Neurogenetic disorders, such as neurofibromatosis type 1 (NF1), are diseases caused by a defect in one or more genes, which can sometimes result in cognitive and motor impairments. Better understanding the neural underpinning of these disorders and how they affect motor and cognitive abilities could contribute to the development of new treatment strategies.
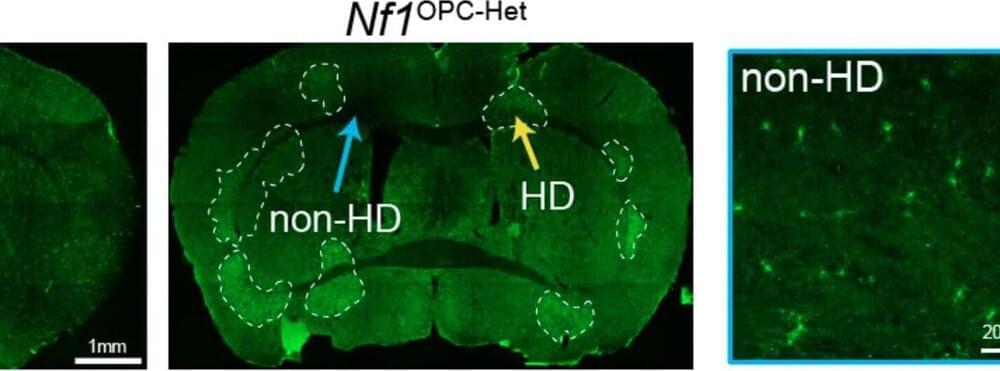
Researchers at Stanford University and Washington University School of Medicine recently performed a study on mice aimed at investigating the impact of Nf1 gene mutations, which cause the NF1 neurogenetic disorder, on oligodendroglial plasticity, an adaptive brain process known to contribute to cognitive and motor functions.
Their findings, published in Nature Neuroscience, provide strong evidence that Nf1 mutations delay the development of oligodendroglia, a type of glial cells that support the functioning of the central nervous system, causing disruptions in motor learning.