Circa 2021 face_with_colon_three
A datacentre that fits in the palm of your hand? However, right now, DNA storage is an expensive chemical process that researchers are trying to make a practical proposal.

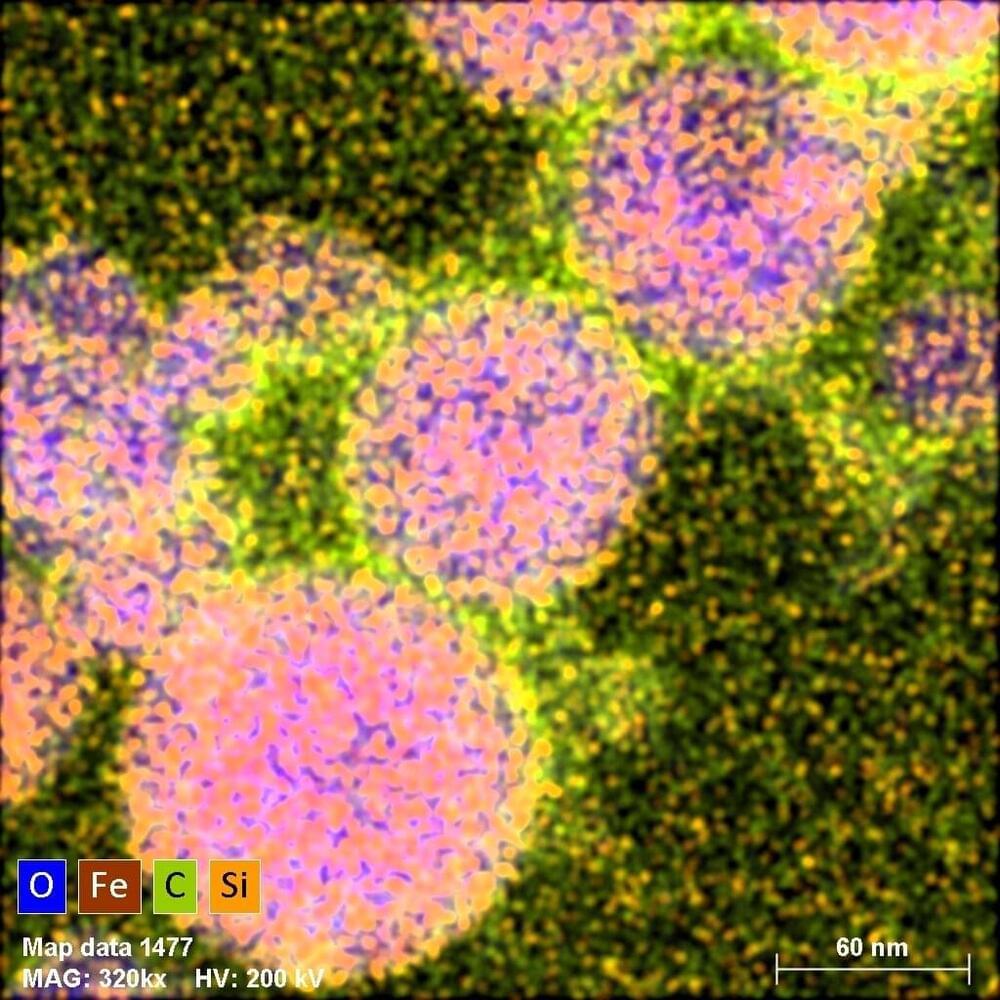
Composite particles with submicron sizes can be produced by irradiating a suspension of nanoparticles with a laser beam. Violent physical and chemical processes take place during irradiation, many of which have been poorly understood to date. Recently completed experiments, carried out at the Institute of Nuclear Physics of the Polish Academy of Sciences in Cracow, have shed new light on some of these puzzles.
When a laser beam strikes agglomerates of nanoparticles suspended in a colloid, events occur that are as dramatic as they are useful. The tremendous increase in temperature leads to the melting together of nanoparticles into a composite particle. A thin layer of liquid next to the heated material rapidly transforms into vapor, and whole sequences of chemical reactions take place under physical conditions that change in fractions of a second. Using this method, called laser melting, scientists from the Institute of Nuclear Physics of the Polish Academy of Sciences (IFJ PAN) in Cracow not only produced new nanocomposites, but also described some of the poorly understood processes responsible for their formation.
“The laser melting process itself, consisting of irradiating particles of material in suspension with unfocused laser light, has been known for years. It is mainly used for the production of single component materials. We, as one of only two research teams in the world, are trying to use this technique to produce composite submicron particles. In this area, the field is still in its infancy, there are still many unknowns, hence our joy that some puzzles that perplexed us have just been unraveled,” says Dr. Żaneta Świątkowska-Warkocka, a professor at IFJ PAN, the co-author of a scientific article just published in the journal Scientific Reports.

Although plant-based polylactic acid (PLA) bioplastic is acclaimed for its biodegradability, it can take quite a long time to degrade if the conditions aren’t quite right. Bearing this fact in mind, Washington State University scientists have devised a way of upcycling it into a 3D-printing resin.
“[PLA] is biodegradable and compostable, but once you look into it, it turns out that it can take up to 100 years for it to decompose in a landfill,” said postdoctoral researcher Yu-Chung Chang, co-corresponding author of the study. “In reality, it still creates a lot of pollution. We want to make sure that when we do start producing PLA on the million-tons scale, we will know how to deal with it.”
To that end, Chang and colleagues developed a process in which an inexpensive chemical known as aminoethanol is used to break down the long chains of molecules that make up PLA. Those chains are rendered into simple monomers, which are the basic building blocks of plastic. The process takes about two days, and can be carried out at mild temperatures.
Solar cell manufacturing just became easier, more efficient, and less costly. A team of researchers at DOE’s Lawrence Berkeley National Laboratory (Berkeley Lab), in collaboration with UC Berkeley, has discovered a unique material that can be used as a simpler approach to solar cell manufacturing, the team reported.
This material is a crystalline solar material with a built-in electric field — also known as “ferroelectricity” — that was reported earlier this year in the journal Science Advances.
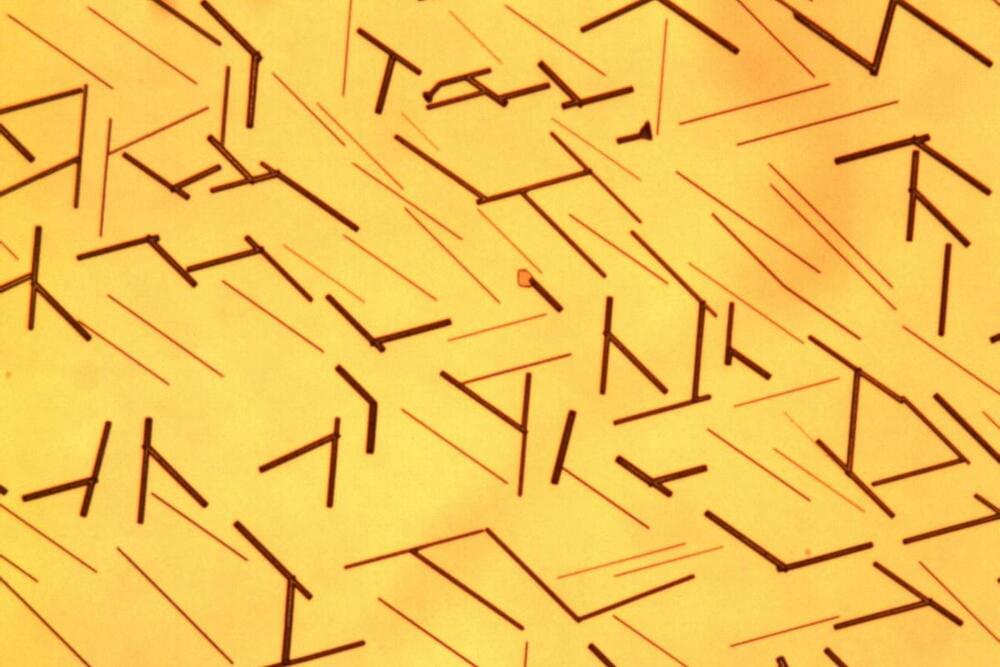
Light microscopy image of nanowires, 100 to 1,000 nanometers in diameter, grown from cesium germanium tribromide (CGB) on a mica substrate. The CGB nanowires are samples of a new lead-free halide perovskite solar material that is also ferroelectric. (Credit: Peidong Yang and Ye Zhang/Berkeley Lab)
Solar panels, also known as photovoltaics, rely on semiconductor devices, or solar cells, to convert energy from the sun into electricity.
To generate electricity, solar cells need an electric field to separate positive charges from negative charges. To get this field, manufacturers typically dope the solar cell with chemicals so that one layer of the device bears a positive charge and another layer a negative charge. This multilayered design ensures that electrons flow from the negative side of a device to the positive side – a key factor in device stability and performance. But chemical doping and layered synthesis also add extra costly steps in solar cell manufacturing.

The human brain is an amazing computing machine. Weighing only three pounds or so, it can process information a thousand times faster than the fastest supercomputer, store a thousand times more information than a powerful laptop, and do it all using no more energy than a 20-watt lightbulb.
Researchers are trying to replicate this success using soft, flexible organic materials that can operate like biological neurons and someday might even be able to interconnect with them. Eventually, soft “neuromorphic” computer chips could be implanted directly into the brain, allowing people to control an artificial arm or a computer monitor simply by thinking about it.
Like real neurons — but unlike conventional computer chips — these new devices can send and receive both chemical and electrical signals. “Your brain works with chemicals, with neurotransmitters like dopamine and serotonin. Our materials are able to interact electrochemically with them,” says Alberto Salleo, a materials scientist at Stanford University who wrote about the potential for organic neuromorphic devices in the 2021 Annual Review of Materials Research.

Too much screen use has been linked to obesity and psychological problems. Now a new study has identified a new problem—a study in fruit flies suggests our basic cellular functions could be impacted by the blue light emitted by these devices. These results are published in Frontiers in Aging.
“Excessive exposure to blue light from everyday devices, such as TVs, laptops, and phones, may have detrimental effects on a wide range of cells in our body, from skin and fat cells, to sensory neurons,” said Dr. Jadwiga Giebultowicz, a professor at the Department of Integrative Biology at Oregon State University and senior author of this study. “We are the first to show that the levels of specific metabolites—chemicals that are essential for cells to function correctly—are altered in fruit flies exposed to blue light.”
“Our study suggests that avoidance of excessive blue light exposure may be a good anti-aging strategy,” advised Giebultowicz.
The thermal radiation emitted by the human body is predominantly in the long-wave infrared region (8–14 μm), which is characterized by low photon energy and low power intensity.
Recently, a research team led by Associate Prof. Lu Xiaowei, Prof. Jiang Peng and Prof. Bao Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) designed a highly sensitive long-wave infrared detector that enables low-power non-contact human-machine interaction.
This study was published in Advanced Materials on July 11.
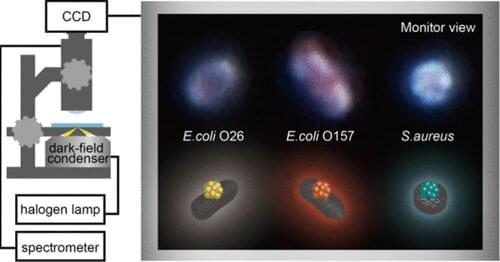
Osaka Metropolitan University scientists have developed a simple, rapid method to simultaneously identify multiple food poisoning bacteria, based on color differences in the scattered light by nanometer-scaled organic metal nanohybrid structures (NHs) that bind via antibodies to those bacteria. This method is a promising tool for rapidly detecting bacteria at food manufacturing sites and thereby improving food safety. The findings were published in Analytical Chemistry.
According to the World Health Organization (WHO), every year food poisoning affects 600 million people worldwide—almost 1 in every 10 people—of which 420,000 die. Bacterial tests are conducted to detect food poisoning bacteria at food manufacturing factories, but it takes more than 48 hours to obtain results due to the time required for a bacteria incubation process called culturing. Therefore, there remains a demand for rapid testing methods to eliminate food poisoning accidents.
Responding to this need, the research team led by Professor Hiroshi Shiigi at the Graduate School of Engineering, Osaka Metropolitan University, utilized the optical properties of organic metal NHs—composites consisting of polyaniline particles that encapsulate a large number of metal nanoparticles—to rapidly and simultaneously identify food poisoning-inducing bacteria called enterohemorrhagic Escherichia coli (E. coli O26 and E. coli O157) and Staphylococcus aureus.
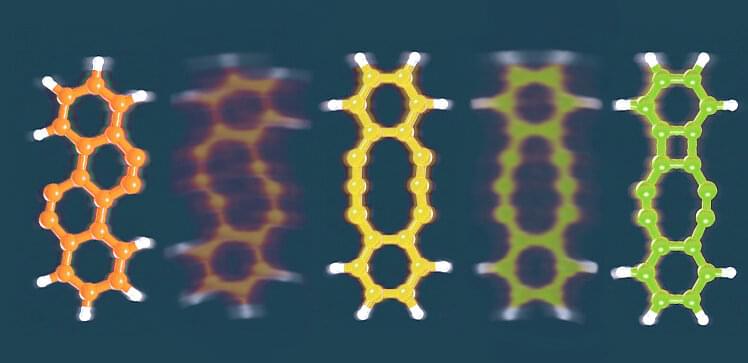
Chemical reactions often produce messy mixtures of different products. Hence, chemists spend a lot of time coaxing their reactions to be more selective to make particular target molecules. Now, an international team of researchers has achieved that kind of selectivity by delivering voltage pulses to a single molecule through an incredibly sharp tip.
“Controlling the pathway of a chemical reaction, depending on the voltage pulses used, is unprecedented and very alluring to chemists,” says KAUST’s Shadi Fatayer.
The team used an instrument that combines scanning tunneling microscopy (STM) and atomic force microscopy (AFM). Both techniques can map out the positions of atoms within individual molecules using a tip that may be just a few atoms wide. But the voltage can also be used to break bonds within a molecule, potentially allowing new bonds to form.