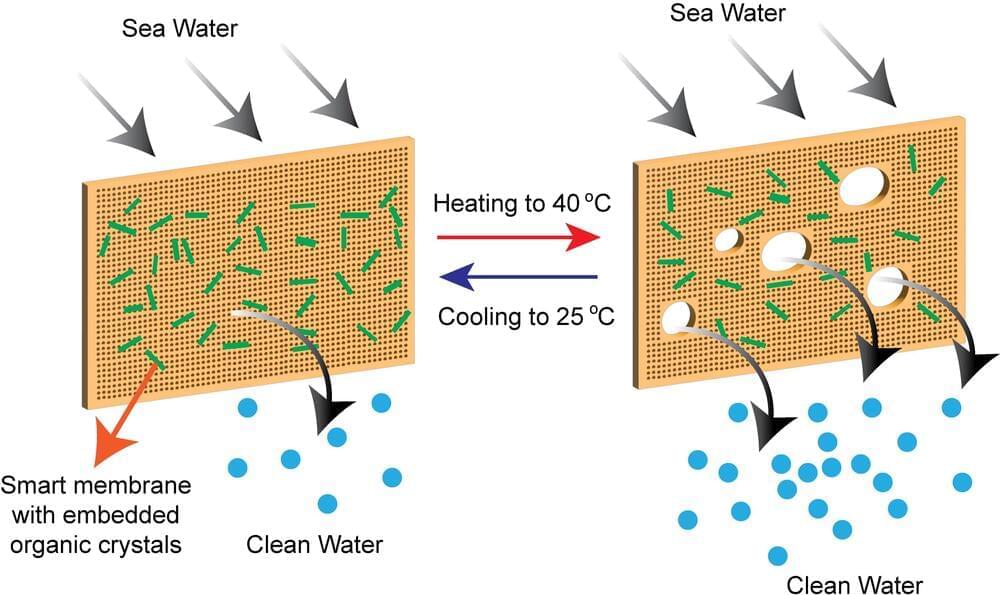
A team of NYU Abu Dhabi (NYUAD) researchers has developed a new kind of self-cleaning, hybrid membrane that provides a solution that overcomes significant challenges that have, until now, limited desalination technologies.
The most energy-efficient desalination technologies are based on membrane desalination. However, the membranes used for desalination are prone to fouling, the accumulation of scale that results in decreased membrane performance, shorter lifespan, and the need for chemical cleaning, which has unknown environmental consequences.
Researchers at NYUAD’s Smart Materials Lab and the Center for Smart Engineering Materials, led by Professor Panče Naumov and Research Scientist Ejaz Ahmed, together with their collaborators from the Institute for Membrane Technology in Italy, created a unique hybrid membrane by utilizing stimuli-responsive materials, thermosalient organic crystals, embedded in polymers. The thermosalient crystals are a new class of dynamic materials that are capable of sudden expansion or motion upon heating or cooling.