Breast cancer in its various forms affects more than 250,000 Americans a year. One particularly aggressive and hard-to-treat type is triple-negative breast cancer (TNBC), which lacks specific receptors targeted by existing treatments. The rapid growth and metastasis of this cancer also make it challenging to manage, leading to limited therapy options and an often poor prognosis for patients.
A promising new approach that uses minuscule tubes to deliver cancer-fighting drugs directly to the tumor site while preserving healthy cells has been developed by Johns Hopkins engineers. The team’s research appeared in Nanoscale.
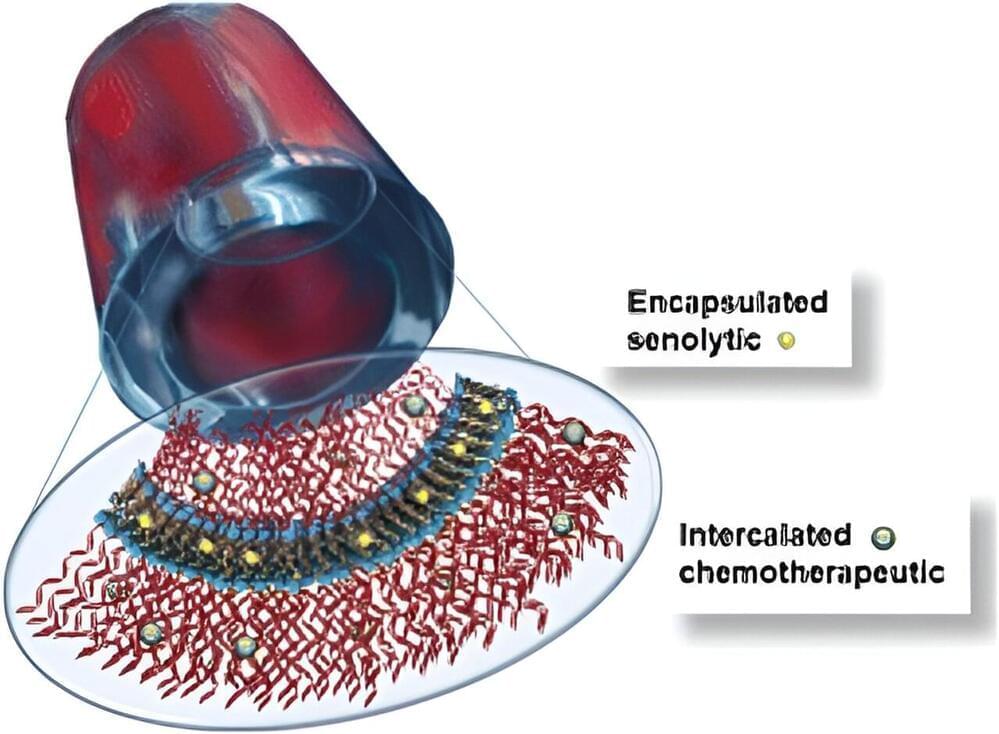
“In this paper, we showed that we can use nanotubes to specifically target both proliferating and senescent TNBC cells with chemotherapeutics and senolytics, killing them without targeting healthy breast cells,” said Efie Kokkoli, professor of chemical and biomolecular engineering, a core researcher at the Johns Hopkins Institute for NanoBioTechnology, and a specialist in engineering targeted nanoparticles for the delivery of cancer therapeutics.