An artificial nerve that is based on a vertical n-type organic electrochemical transistor with a gradient-intermixed bicontinuous structure can operate at high frequencies and mimic basic conditioned reflex behaviour in animals.

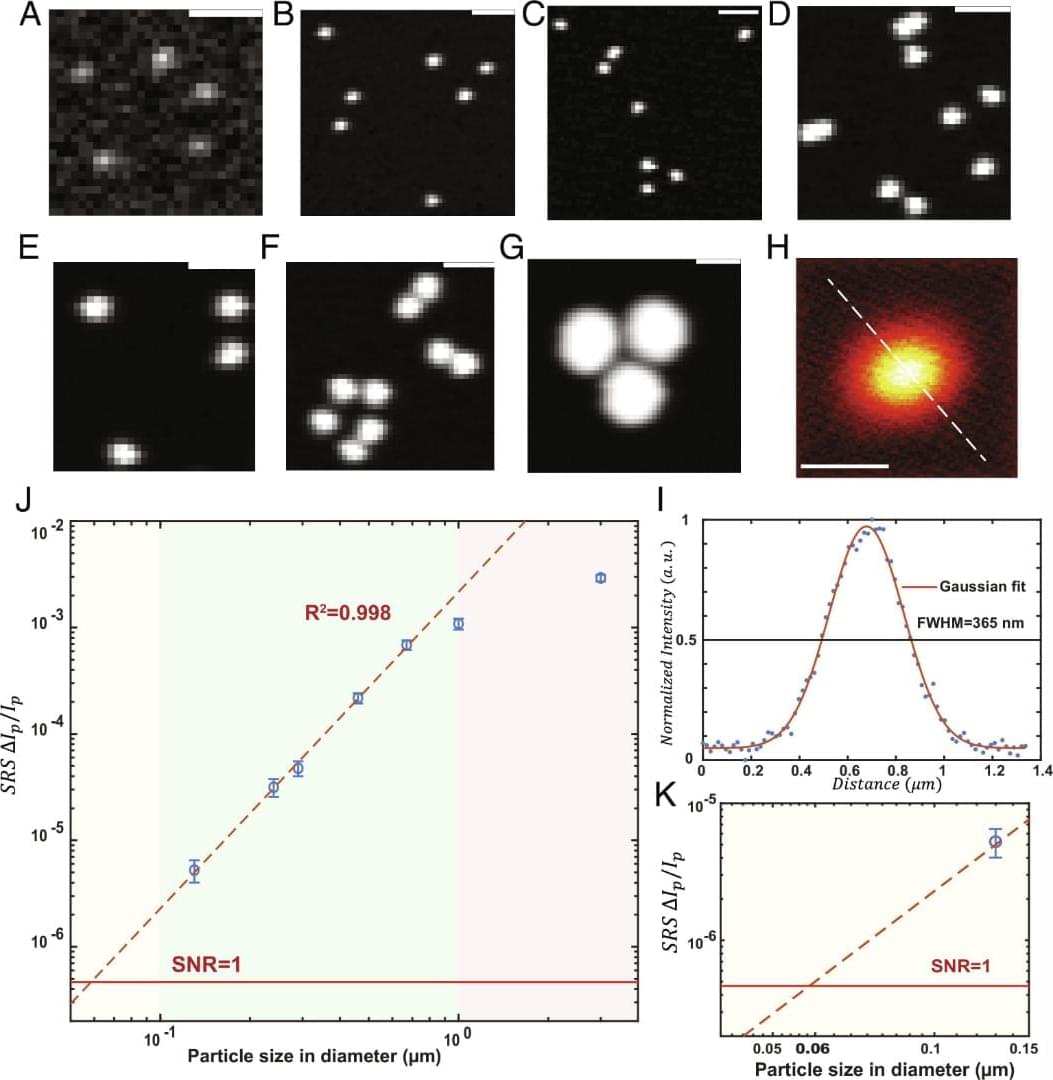
The shape is another important morphological feature that matters as a critical aspect of nanotoxicity. Studies have shown that shape plays a role in determining the cellular uptake of micro-nano particles (65, 66). SRS images of plastic particles confirmed the existence of shape diversity for micro-nano plastics in bottled water. To account for the shape of plastic particles in a statistical manner, we measure the aspect ratio of individual particles above the diffraction limit (Fig. 6 H). The aspect ratio is widely acknowledged in nanotoxicology studies (67, 68). The aspect ratio of the plastic particles detected ranges from 1 to 6, and the average aspect ratio for particles is around 1.7. Fig. 6 I–M provides a pictorial view of how the aspect ratio is related to the particle shape. Particles with an aspect ratio of above 3 are most likely to be fibrous in shape, while particles with an aspect ratio of below 1.4 will be largely spherical. Shape variation on plastic particles has been found in all polymers detected, confirming the widely recognized idea that real-world micro-nano plastics have diverse morphological prosperities. This dimension is hard to be resembled by engineered polymer nanoparticles commonly studied in research laboratories, and the toxicological consequences pertaining to real-life plastic particle exposures and their differing physicochemical properties (i.e., size, shape) have yet to be determined.
It’s about my paper.
Dissolving polymers with organic solvents is the essential process in the research and development of polymeric materials, including polymer synthesis, refining, painting, and coating. Now more than ever recycling plastic waste is a particularly imperative part of reducing carbon produced by the materials development processes.
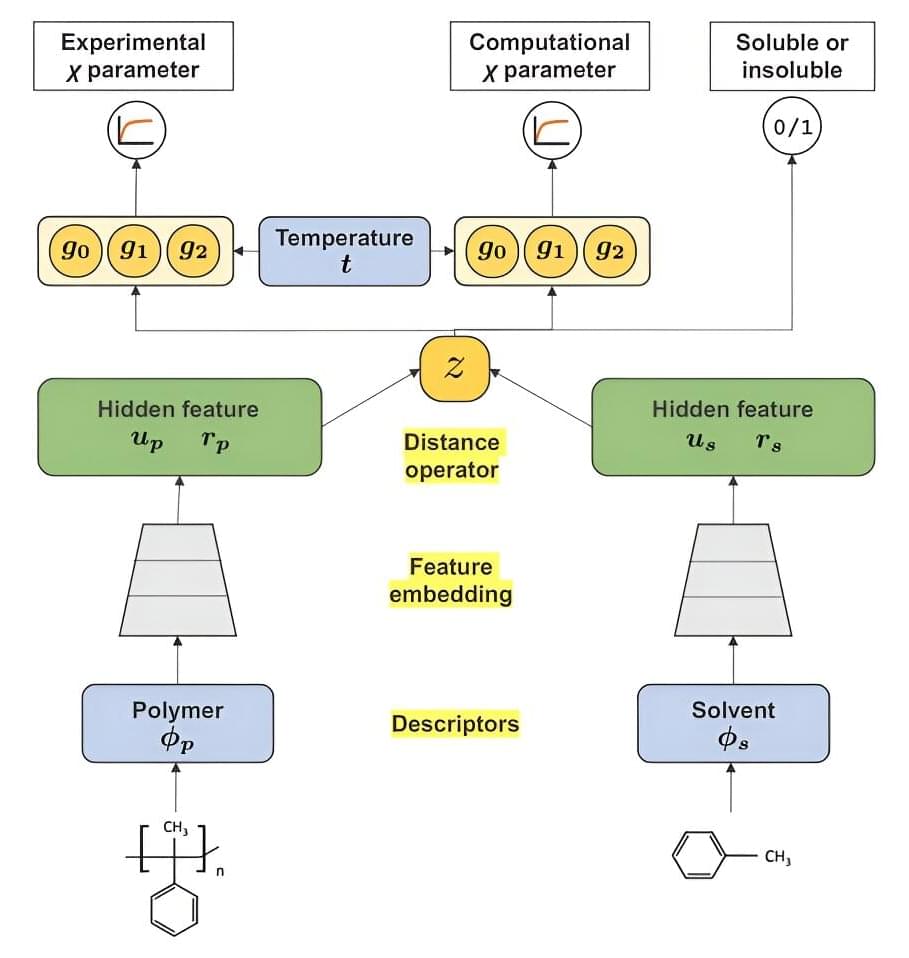
Polymers, in this instance, refer to plastics and plastic-like materials that require certain solvents to be able to effectively dissolve and therefore become recyclable, though it’s not as easy as it sounds. Utilizing Mitsubishi Chemical Group’s (MCG) databank of quantum chemistry calculations, scientists developed a novel machine learning system for determining the miscibility of any given polymer with its solvent candidates, referred to as χ (chi) parameters.
This system has enabled scientists to overcome the limitations arising from a limited amount of experimental data on the polymer-solvent miscibility by integrating massive data produced from the computer experiments using high-throughput quantum chemistry calculations.

University of Arizona astronomers have learned more about a surprisingly mature galaxy that existed when the universe was just less than 300 million years old—just 2% of its current age.
Observed by NASA’s James Webb Space Telescope, the galaxy—designated JADES-GS-z14-0—is unexpectedly bright and chemically complex for an object from this primordial era, the researchers said. This provides a rare glimpse into the universe’s earliest chapter.
The findings, published in the journal Nature Astronomy, build upon the researchers’ previous discovery, reported in 2024, of JADES-GS-z14-0 as the most distant galaxy ever observed. While the initial discovery established the galaxy’s record-breaking distance and unexpected brightness, this new research delves deeper into its chemical composition and evolutionary state.

A mysterious phenomenon at the center of our galaxy could be the result of a different type of dark matter.
Dark matter, the mysterious form of unobserved matter which could make up 85% of the mass of the known universe, is one of science’s biggest manhunts.
In this first of its kind study, scientists have taken a step closer to understanding the elusive mystery matter. They believe a reimagined candidate for dark matter could be behind unexplained chemical reactions taking place in the Milky Way.
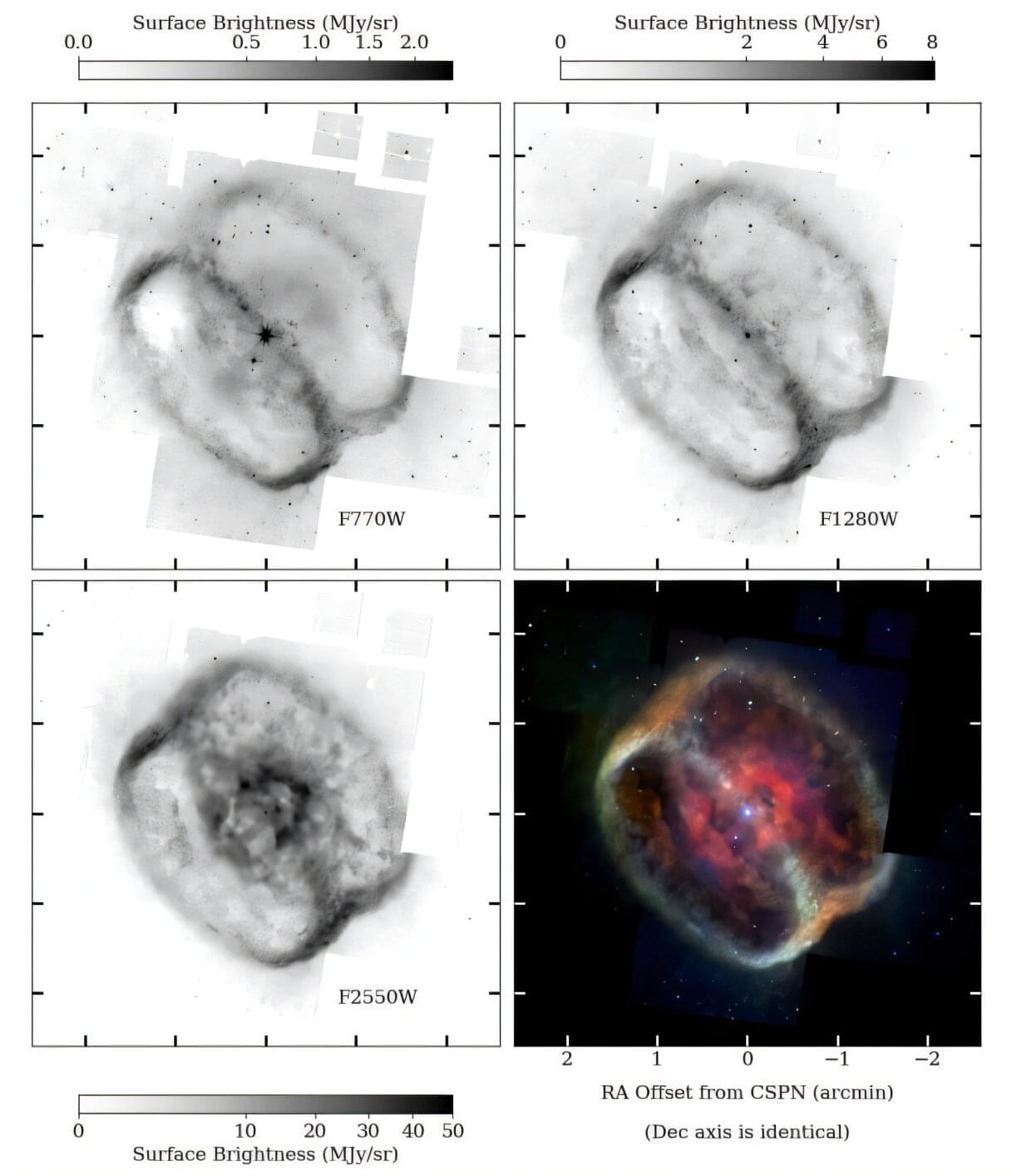
Using the James Webb Space Telescope (JWST), astronomers have observed enigmatic rings in the planetary nebula NGC 1,514, visible in the mid-infrared band. Results of the new observations, published Feb. 28 on the arXiv pre-print server, shed more light on the properties and nature of these rings.
Planetary nebulae (PNe) are expanding shells of gas and dust that have been ejected from a star during the process of its evolution from a main sequence star into a red giant or white dwarf. They are relatively rare, but are important for astronomers studying the chemical evolution of stars and galaxies.
NGC 1,514 (also known as Crystal Ball Nebula) is a large and complex elliptical planetary nebula at a distance of about 1,500 light years away. It originated from a binary star designated HD 281679. The bright, visible component of the system is a giant star of spectral type A0III, while the nebula-generating companion is now a hot, sub-luminous O-type star.
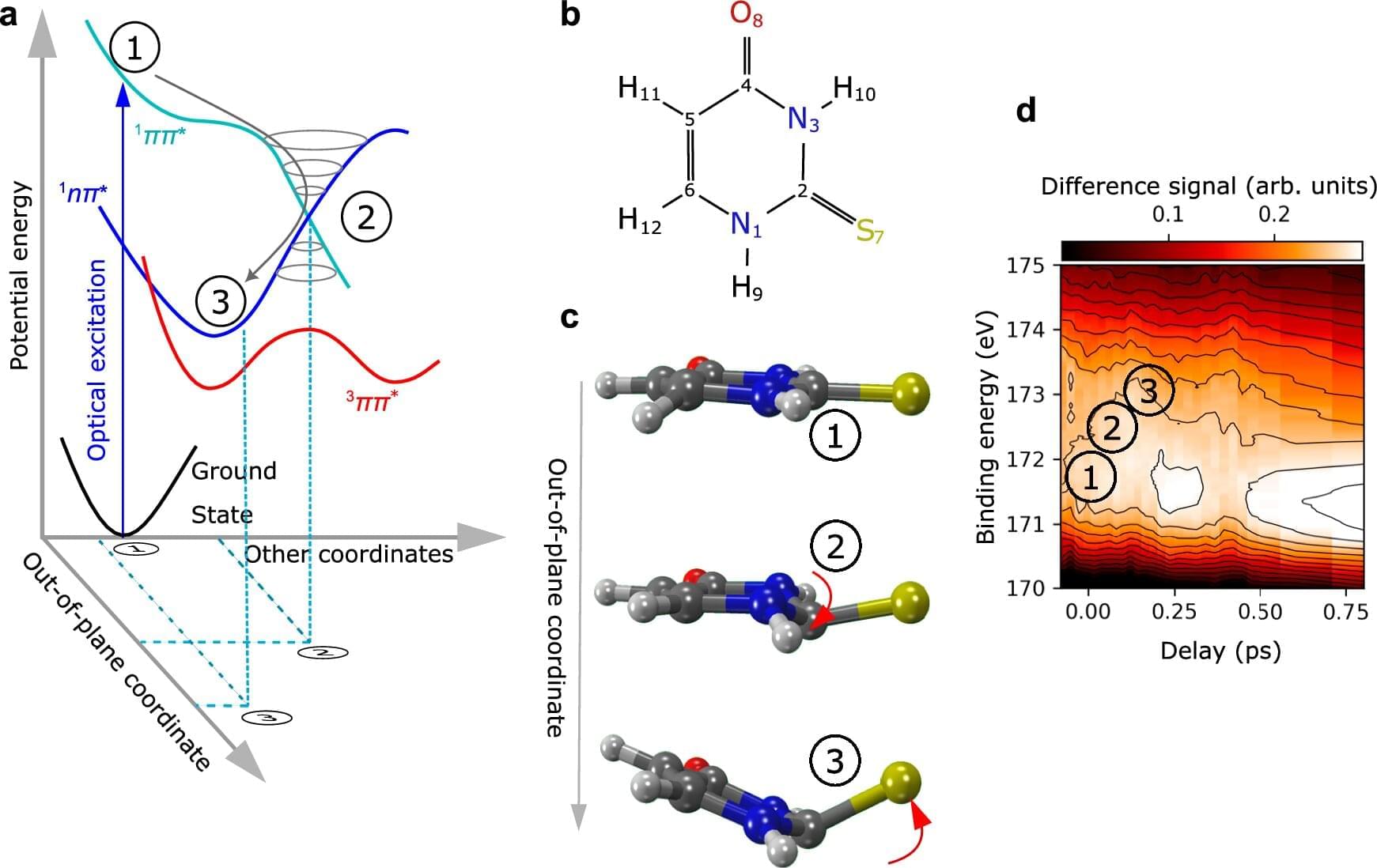
Many biologically important molecules change shape when stimulated by UV radiation. Although this property can also be found in some drugs, it is not yet well understood. Using an innovative technique, an international team involving researchers from Goethe University Frankfurt, the European XFEL in Schenefeld and the Deutschen Elektronen-Synchrotron DESY in Hamburg has elucidated this ultra-fast process, and made it visible in slow motion, with the help of X-ray light. The method opens up exciting new ways of analyzing many other molecules.
The study is published in the journal Nature Communications.
“We investigated the molecule 2-thiouracil, which belongs to a group of pharmaceutically active substances based on certain DNA building blocks, the nucleobases,” says the study’s last author Markus Gühr, the head of DESY’s free-electron laser FLASH and Professor of Chemistry at University of Hamburg. 2-thiouracil and its chemically related active substances have a sulfur atom, which gives the molecules its unusual, medically relevant properties.

Researchers from Japan and Taiwan have made a groundbreaking discovery, demonstrating for the first time that helium—long considered chemically inert—can bond with iron under extreme pressure. Using a laser-heated diamond anvil cell, they observed this unexpected interaction, suggesting that vast amounts of helium may be present in the Earth’s core. This finding challenges long-held theories about the planet’s internal structure and history and could provide new insights into the primordial nebula from which our solar system originated.
Volcanic eruptions primarily release rocks and minerals, but they can also emit traces of a rare gas known as primordial helium. Unlike the more common isotope, helium-4 (⁴He), which consists of two protons and two neutrons and is continuously produced by radioactive decay, primordial helium—helium-3 (³He)—contains only one neutron and is not formed on Earth. Its presence offers valuable clues about the planet’s deep interior and its connection to cosmic origins.
Given the occasionally high 3 He/4He ratios found in volcanic rocks, especially in Hawaii, researchers have long believed there are primordial materials containing 3 He deep within the mantle. However, graduate student Haruki Takezawa and members of Professor Kei Hirose’s group from the University of Tokyo’s Department of Earth and Planetary Science have now challenged this view with a new take on a familiar experiment — crushing things.
The world’s demand for alternative fuels and sustainable chemical products has prompted many scientists to look in the same direction for answers: converting carbon dioxide (CO2) into carbon monoxide (CO).
But the labs of Yale chemists Nilay Hazari and James Mayer have a different chemical destination in mind. In a new study, Hazari, Mayer, and their collaborators present a new method for transforming CO2 into a chemical compound known as formate — which is used primarily in preservatives and pesticides, and which may be a potential source of more complex materials.