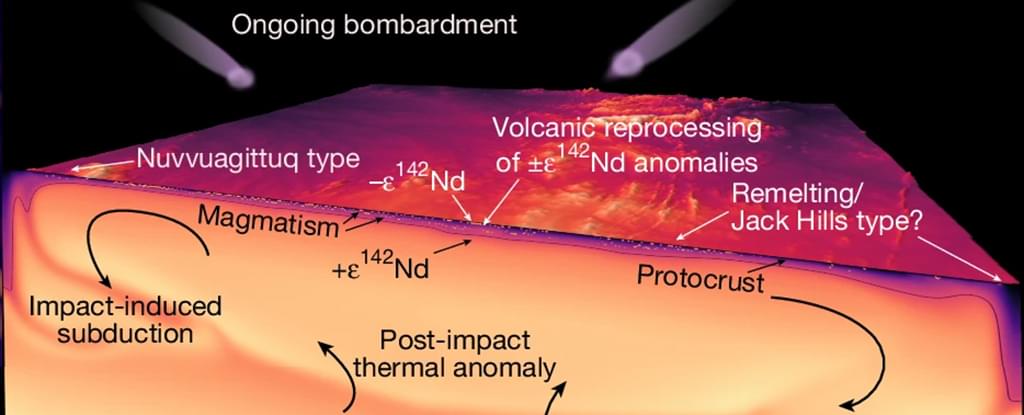
Geologists have made certain assumptions about how the crust making up our planet’s earliest surface formed, but a new study has found that Earth’s very first protocrust was surprisingly similar to the shell of solid rock in place today.
It may mean a complete rethink of how Earth’s coat transitioned from a skin of boiling magma to the shifting armor of tectonic plates we now live on, according to the international team of researchers behind the study.
“Scientists have long thought that tectonic plates needed to dive beneath each other to create the chemical fingerprint we see in continents,” says geochemist Simon Turner, from Macquarie University in Australia.