Within the last century different ways have been developed to fight cancer. The most recent type of therapy includes immunotherapy. Immunotherapy activates the immune system to recognize and target the tumor that was initially undetectable. The most common immunotherapy treatments include anti-programmed death-1 (anti-PD-1) and anti-cytotoxic T lymphocyte-associated antigen (anti-CTLA-4). The two therapies are known as checkpoint inhibitors because they block cell signaling between immune cells and cancer which allow immune cells to be activated and kill the tumor. For their work on both of these therapies, Dr. James Allison and Dr. Tasuku Honjo were awarded the Nobel Prize in Physiology or Medicine in 2018. Since the discovery of these checkpoint inhibitors, they have been used in multiple settings as single-agent therapies or in combination against many different cancers.
The use of checkpoint inhibitors before therapy or as a neoadjuvant has grown as a possible treatment in various cancer types. According to researchers at the Bloomberg-Kimel Institute for Cancer Immunotherapy and the Johns Hopkins Kimmel Cancer Center medicine, checkpoint inhibitors have just touched the surface in neoadjuvant immunotherapy. Researchers including Suzanne Topalian, Director of Johns Hopkins Melanoma/Skin Cancer Program, believe that cancer immunotherapy has a lot of rich untapped potential that can be used to further improve treatments.
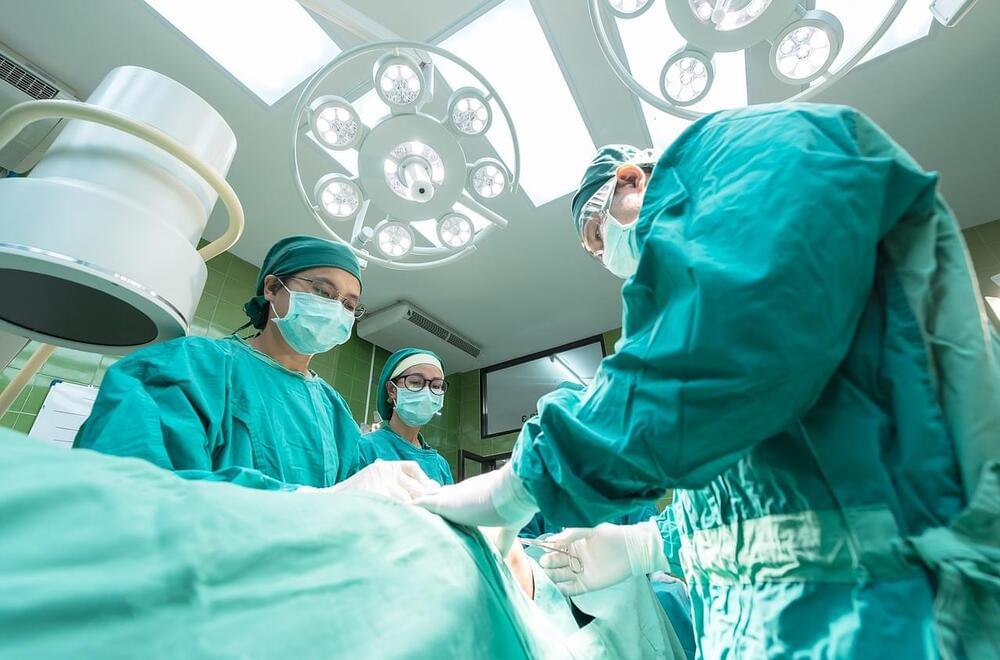
Neoadjuvant immunotherapy, or therapy prior to surgery, has since been tested in various clinical trials. In some tumor types this form of therapy has resulted in complete tumor eradication. The report published in Cancer Cell highlights successful clinical trials in cancers such as lung, triple-negative breast, skin, and gastrointestinal. Although this therapy is still highly underdeveloped, the US Food and Drug Administration (FDA) has already approved neoadjuvant immunotherapy in triple-negative breast and lung cancer. It is expected that more approvals will follow for other cancer types.