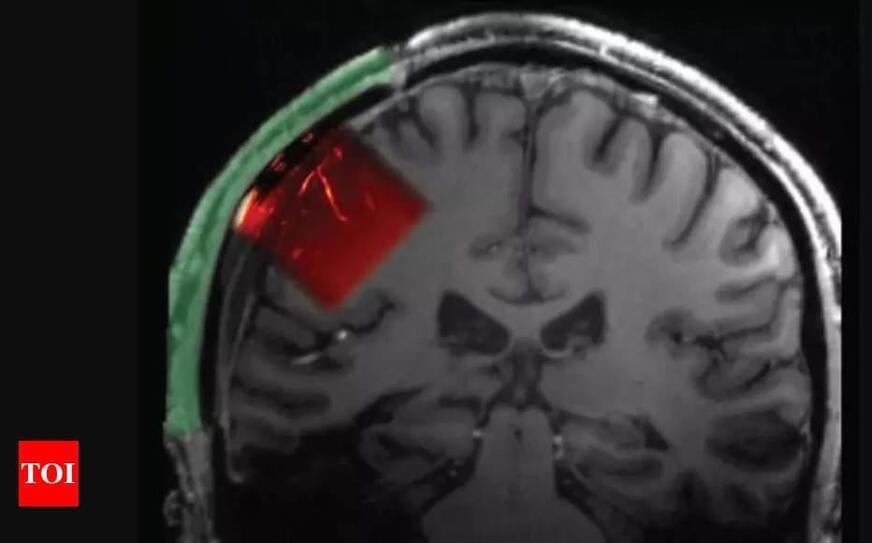
Science and Technology: Some robots could be “eaten” so they could walk around inside the body and perform tests or surgeries from the inside out; or administer medications.
Robots made of several nanorobots joined together could assemble and reassemble themselves inside the body even after being…
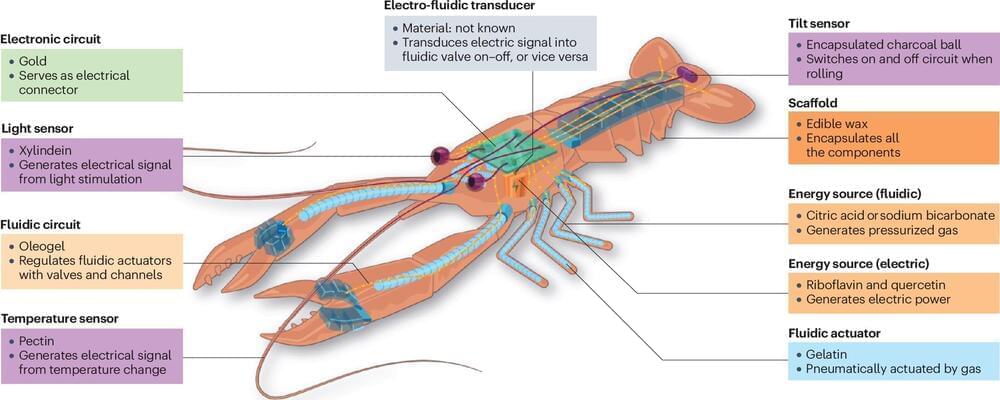
Robots and food have long been distant worlds: Robots are inorganic, bulky, and non-disposable; food is organic, soft, and biodegradable. Yet, research that develops edible robots has progressed recently and promises positive impacts: Robotic food could reduce electronic waste, help deliver nutrition and medicines to people and animals in need, monitor health, and even pave the way to novel gastronomical experiences.
But how far are we from having a fully edible robot for lunch or dessert? And what are the challenges? Scientists from the RoboFood project, based at EPFL, address these and other questions in a perspective article in the journal Nature Reviews Materials.
“Bringing robots and food together is a fascinating challenge,” says Dario Floreano, director of the Laboratory of Intelligent Systems at EPFL and first author of the article. In 2021, Floreano joined forces with Remko Boom from Wageningen University, The Netherlands, Jonathan Rossiter from the University of Bristol, UK, and Mario Caironi from the Italian Institute of Technology, to launch the project RoboFood.