Hong Kong to unleash mosquito-killing robot dogs to combat Chikungunya virus.
Hong Kong plans to deploy robot dogs to combat the chikungunya virus through innovative insecticide spraying in hard-to-reach areas.




The US Food and Drug Administration (FDA) has approved Tonix Pharmaceuticals’ Tonmya (cyclobenzaprine HCl, formerly known as TNX-102 SL), a novel treatment form for fibromyalgia.1 The drug is now the first in a new category of non-opioid analgesics for fibromyalgia and the first new medication for this disorder in 15 years.
The FDA’s approval cited efficacy from 2 double-blind, randomized, placebo-controlled, phase 3 clinical trials of almost 1,000 patients that evaluated Tonmya as treatment for fibromyalgia. Across both phase 3 trials, Tonmya significantly reduced daily pain scores compared with placebo at the primary endpoint of 14 weeks. In these trials, a greater percentage of patients taking Tonmya experienced a clinically meaningful (≥30%) improvement in their pain after 3 months, as compared with placebo. Across phase 3 clinical trials with over 1,400 patients evaluated, Tonmya was generally well tolerated. The most common adverse events (incidence ≥2%) included oral hypoesthesia, oral discomfort, abnormal product taste, somnolence, oral paresthesia, oral pain, fatigue, dry mouth, and aphthous ulcer.
Psychiatric Times is the connection to Psychiatry and Mental Health, featuring clinical updates, expert views, and research news in multimedia formats.

The US FDA has granted De Novo marketing authorization for ArteraAI Prostate (Artera, Los Altos, California), a novel artificial intelligence (AI)-powered risk-stratification tool for patients with nonmetastatic prostate cancer.
The authorization means the digital pathology software tool is recognized as an FDA-regulated Software as a Medical Device, Artera explained in a press release.
De Novo authorization provides a marketing pathway to classify low-or moderate-risk novel medical devices. The De Novo authorization for this specific test establishes a new product code category for future AI-powered digital pathology risk-stratification tools and enables implementation at the point of diagnosis at qualified US pathology labs, the company said.
The test analyzes digital pathology images from patients’ biopsy slides to predict long-term outcomes, such as 10-year risk for metastasis and mortality. This can help direct treatment decisions.
The FDA’s De Novo authorization for the tool establishes a new product code category for future AI-powered digital pathology risk-stratification tools.

Patients arriving at appointments with researched information is not new, but artificial intelligence (AI) tools such as ChatGPT are changing the dynamics.
Their confident presentation can leave physicians feeling that their expertise is challenged. Kumara Raja Sundar, MD, a family medicine physician at Kaiser Permanente Burien Medical Center in Burien, Washington, highlighted this trend in a recent article published in JAMA.
A patient visited Sundar’s clinic reporting dizziness and described her symptoms with unusual precision: “It’s not vertigo, but more like a presyncope feeling.” She then suggested that the tilt table test might be useful for diagnosis.
Occasionally, patient questions reveal subtle familiarity with medical jargon. This may indicate that they either have relevant training or have studied the subject extensively.
(Artificial Intelligence is the science of making machines do things that would require intelligence if done by men — Marvin Minsky. Google helps you gain information with a search engine. AI helps you gain information through algorithms. It is the same thing. However people profit from ignorance).
Patients are showing up with ChatGPT-generated diagnoses, challenging physicians to balance empathy, evidence, and authority in the exam room.
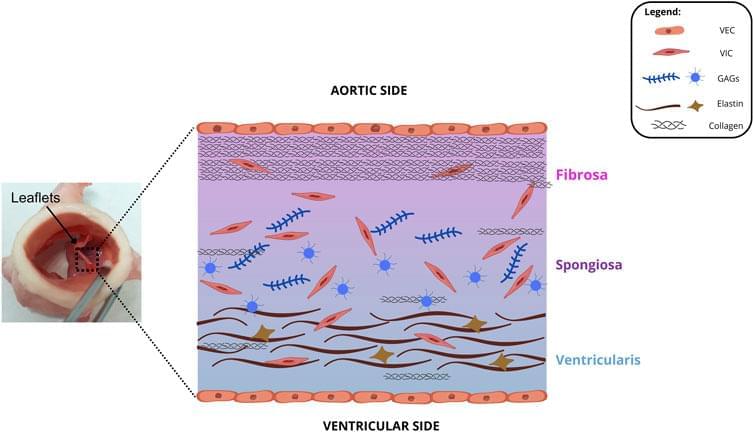
Prosthetic heart valves (PHV) have been studied for around 70 years. They are the best alternative to save the life of patients with cardiac valve diseases. However, current PHVs may still cause significant disadvantages to patients. In general, native heart valves show complex structures and reproducing their functions challenges scientists. Valve repair and replacement are the options to heal heart valve diseases (VHDs), such as stenosis and regurgitation, which show high morbidity and mortality worldwide. Valve repair contributes to the performance of cardiac cycles. However, it fails to restore valve anatomy to its normal condition. On the other hand, replacement is the only alternative to treat valve degeneration. It may do so by mechanical or bioprosthetic valves. Although prostheses may restructure patients’ cardiac cycle, both prostheses may show limitations and potential disadvantages, such as mechanical valves causing thrombogenicity or bioprosthetic valves, calcification. Thus, prostheses require constant improvements to remedy these limitations. Although the design of mechanical valve structures has improved, their raw materials cause great disadvantages, and alternatives for this problem remain scarce. Cardiac valve tissue engineering emerged 30 years ago and has improved over time, e.g., xenografts and fabricated heart valves serving as scaffolds for cell seeding. Thus, this review describes cardiac valve substitutes, starting with the history of valvular prosthesis transplants and ending with some perspectives to alleviate the limitations of artificial valves.
GRAPHICAL ABSTRACT
Scientists have for the first time ever filmed a crucial step that leads to new human life – cracking open a ‘black box’ of early development, and potentially helping with fertility treatments.
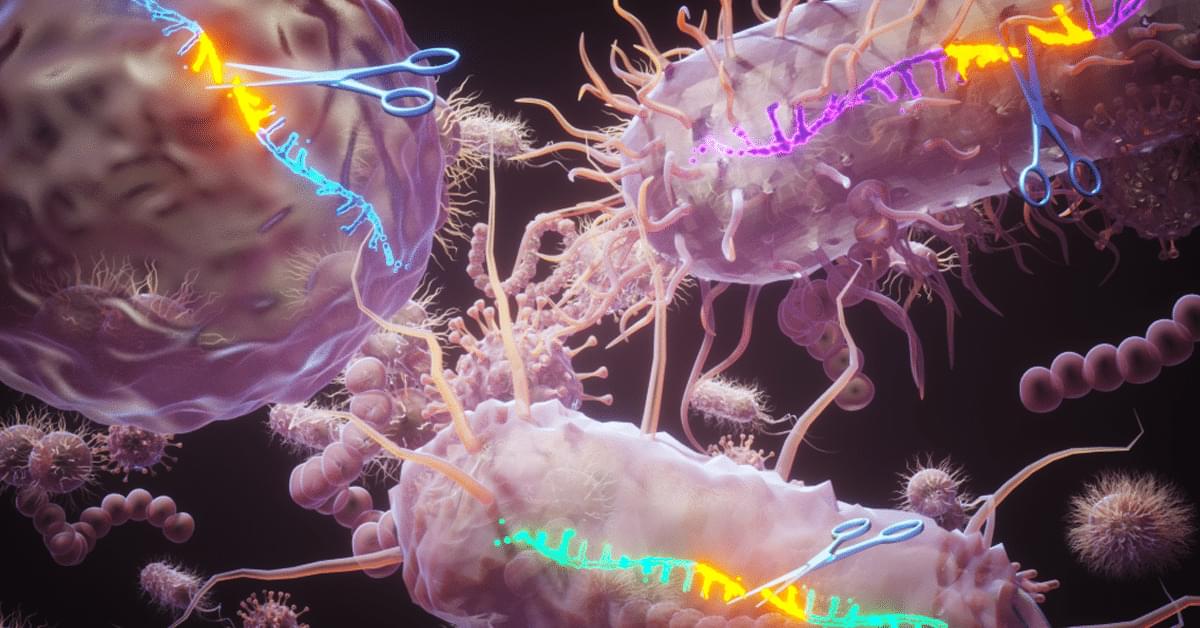
A research team headed by the University of Zurich has developed a powerful new method to precisely edit DNA by combining cutting-edge genetic engineering with artificial intelligence. This technique opens the door to more accurate modeling of human diseases and lays the groundwork for next-generation gene therapies.
Precise and targeted DNA editing by small point mutations as well as the integration of whole genes via CRISPR/Cas technology has great potential for applications in biotechnology and gene therapy. However, it is very important that the so-called gene scissors do not cause any unintended genetic changes, but maintain genomic integrity to avoid unintended side effects. Normally, double-stranded breaks in the DNA molecule are accurately repaired in humans and other organisms. But occasionally, this DNA end joining repair results in genetic errors.
Gene editing with greatly improved precision Now, scientists from the University of Zurich (UZH), Ghent University in Belgium and the ETH Zurich have developed a new method which greatly improves the precision of genome editing. Using artificial intelligence (AI), the tool called Pythia predicts how cells repair their DNA after it is cut by gene editing tools such as CRISPR/Cas9. “Our team developed tiny DNA repair templates, which act like molecular glue and guide the cell to make precise genetic changes,” says lead author Thomas Naert, who pioneered the technology at UZH and is currently a postdoc at Ghent University.
Page description.