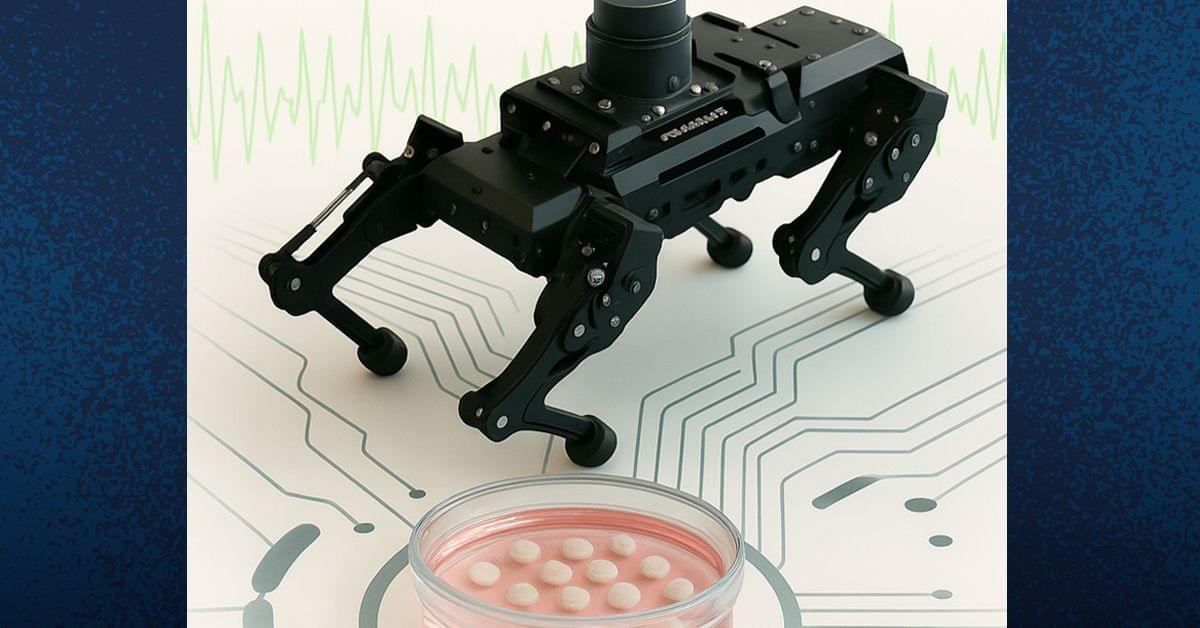
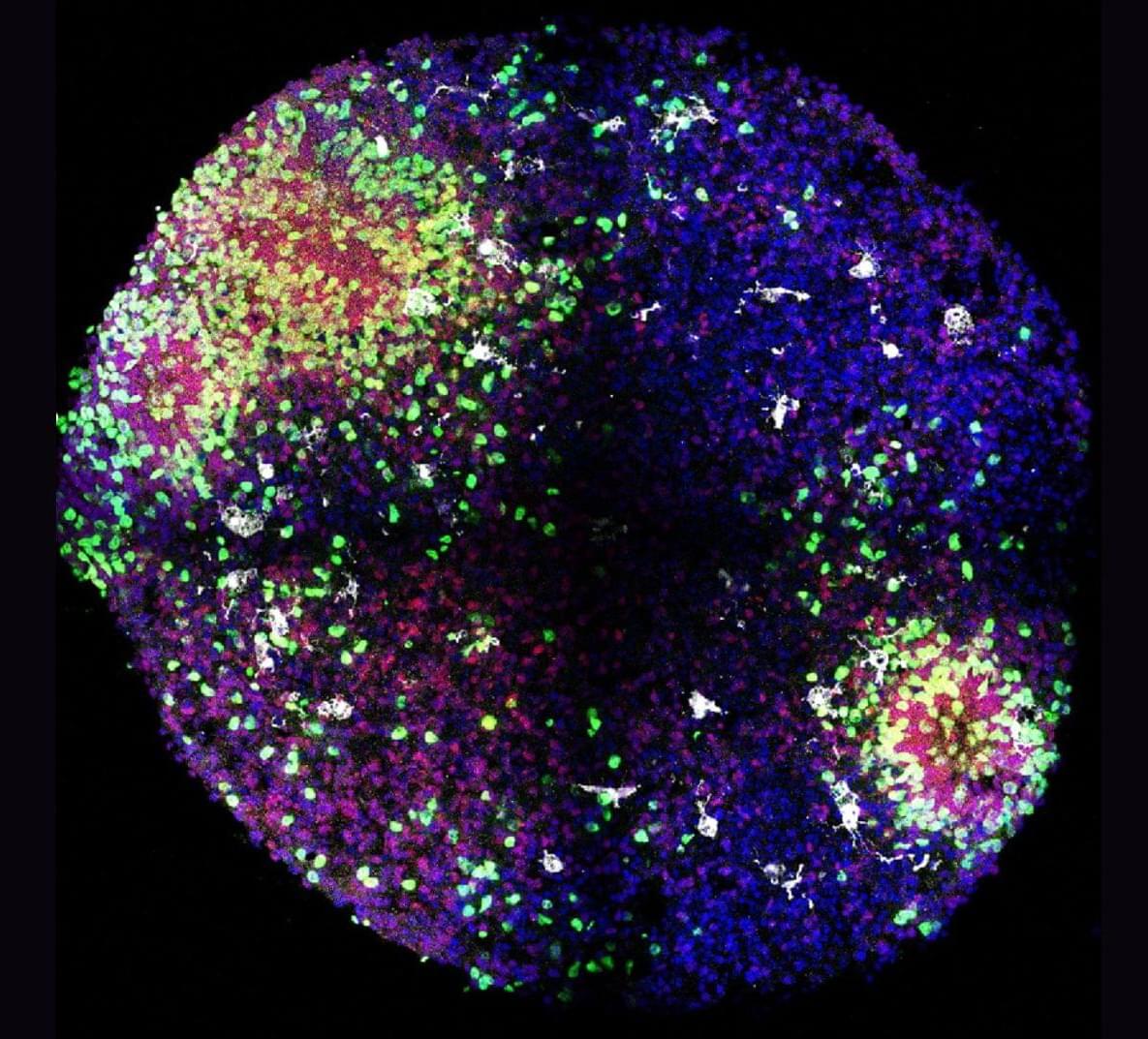
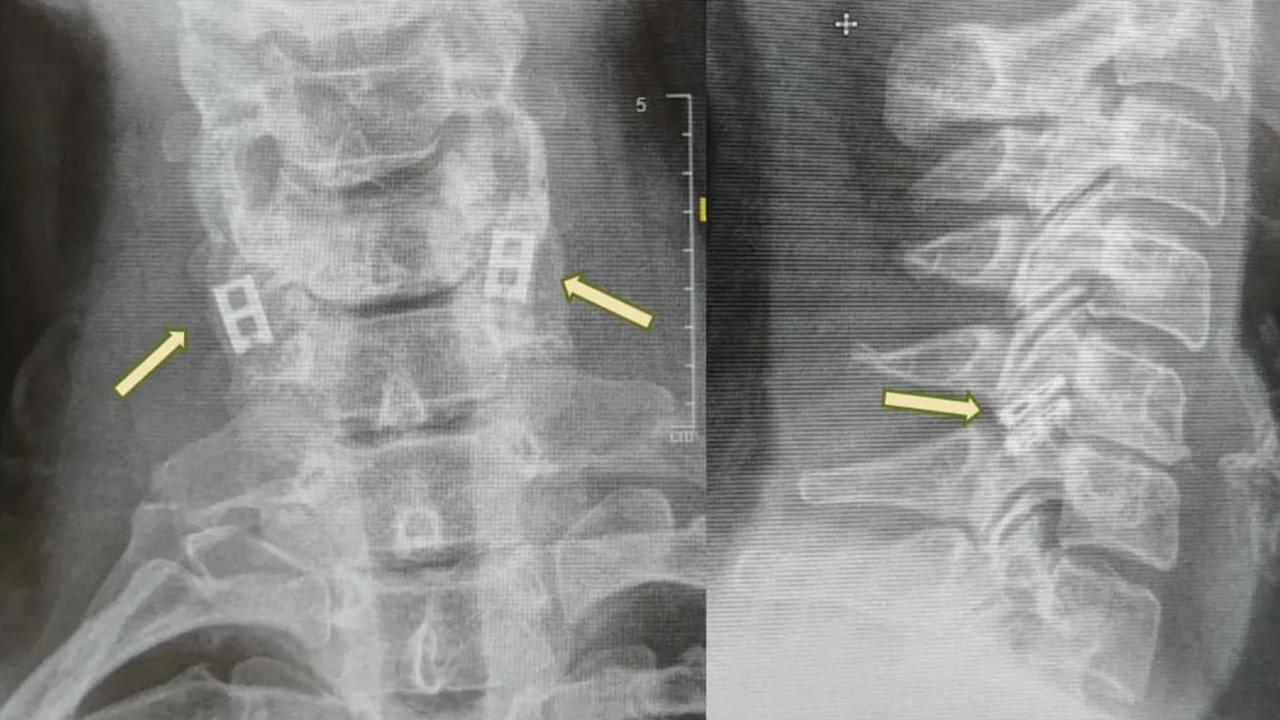
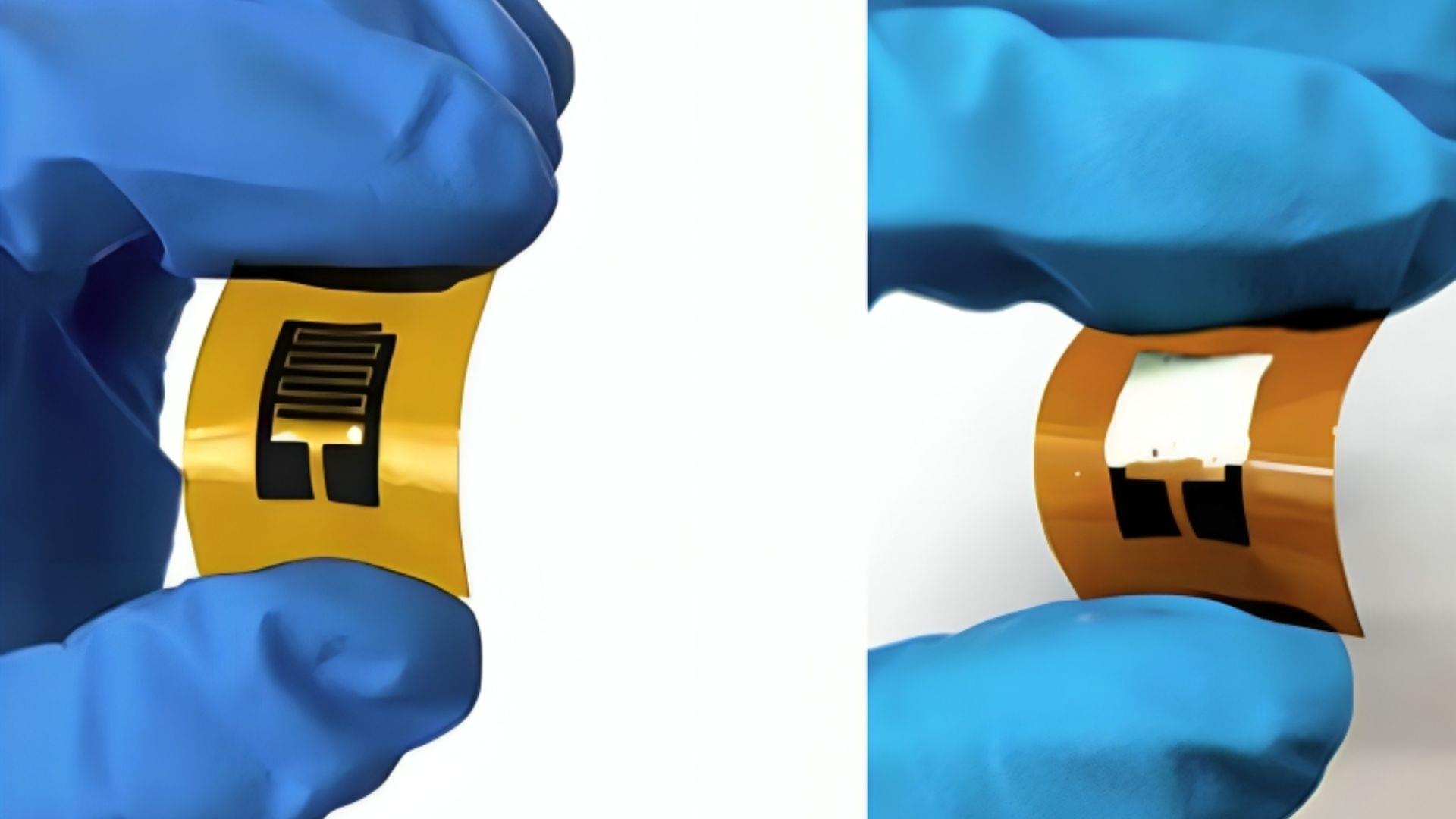
Researchers from University of California San Diego Sanford Stem Cell Institute have developed a novel method to stimulate and mature human brain organoids using graphene, a one-atom-thick sheet of carbon. Published in Nature Communications, the study introduces Graphene-Mediated Optical Stimulation (GraMOS), a safe, non-genetic, biocompatible, non-damaging way to influence neural activity over days to weeks. The approach accelerates brain organoid development — especially important for modeling age-related conditions like Alzheimer’s disease — and even allows them to control robotic devices in real time.
“This is a game-changer for brain research,” said Alysson Muotri, Ph.D., corresponding author, professor of pediatrics, and director of the UC San Diego Sanford Stem Cell Institute Integrated Space Stem Cell Orbital Research Center. “We can now speed up brain organoid maturation without altering their genetic code, opening doors for disease research, brain–machine interfaces and other systems combining living brain cells with technology.”