DNA sequestered in circulating platelets could inform early tumor diagnosis


Scientists from Trinity College Dublin have discovered that electrically stimulating macrophages—one of the immune systems key players—can reprogram them in such a way as to reduce inflammation and encourage faster, more effective healing in disease and injury.
This breakthrough uncovers a potentially powerful new therapeutic option, with further work ongoing to delineate the specifics.
Macrophages are a type of white blood cell with several high-profile roles in our immune system. They patrol around the body, surveying for bugs and viruses, as well as disposing of dead and damaged cells, and stimulating other immune cells —kicking them into gear when and where they are needed.

Water cure: The study found that common shrews shrink their brains in winter not by losing cells, but by losing water.
Brain scans: The team used MRI scanning, the same technology used in hospitals, to peer inside the brains of live shrews across seasons.
What humans can learn: Brain shrinkage in humans is typically a sign of disease, like Alzheimer’s. But shrews can shrink their brain without compromising function or causing damage. Shrews could become a model system for exploring potential pathways for medica treatment of human brain disease.
Knowing how shrews loose brain volume over winter is the first step to understanding how they reverse this loss and regrow healthy brains in summer.

Our bodies are colonized by a teeming, ever-changing mass of microbes that help power countless biological processes. Now, a new study has identified how these microorganisms get to work shaping the brain before birth.
Researchers at Georgia State University studied newborn mice specifically bred in a germ-free environment to prevent any microbe colonization. Some of these mice were immediately placed with mothers with normal microbiota, which leads to microbes being transferred rapidly.
That gave the study authors a way to pinpoint just how early microbes begin influencing the developing brain. Their focus was on the paraventricular nucleus (PVN), a region of the hypothalamus tied to stress and social behavior, already known to be partly influenced by microbe activity in mice later in life.

Nutrition is one of the most influential environmental factors in both taxonomical shifts in gut microbiota as well as in the development of type 2 diabetes mellitus (T2DM). Emerging evidence has shown that the effects of nutrition on both these parameters is not mutually exclusive and that changes in gut microbiota and related metabolites such as short-chain fatty acids (SCFAs) and branched-chain amino acids (BCAAs) may influence systemic inflammation and signaling pathways that contribute to pathophysiological processes associated with T2DM. With this background, our review highlights the effects of macronutrients, carbohydrates, proteins, and lipids, as well as micronutrients, vitamins, and minerals, on T2DM, specifically through their alterations in gut microbiota and the metabolites they produce.
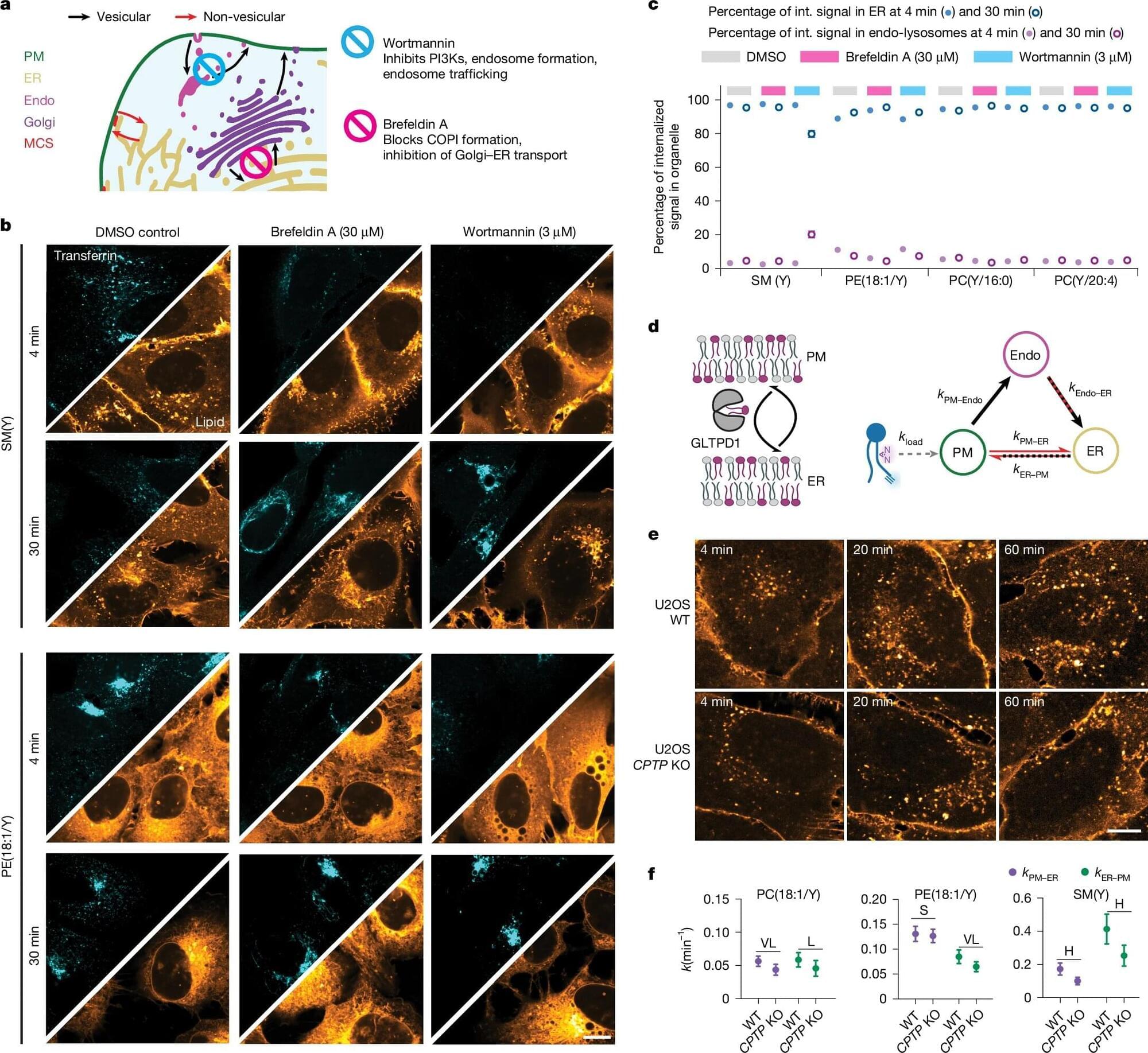
Max Planck Institute of Molecular Cell Biology and Genetics led a study showing that directional, non-vesicular lipid transport drives fast, species-selective lipid sorting, outpacing slower, less specific vesicular trafficking, and yielding a quantitative map of retrograde lipid transport in cells.
Thousands of lipid species occupy distinct organelle membranes, with task differences that determine cellular function. Gaps in live-cell imaging capabilities have limited clarity on how individual lipids move between organelles to maintain those tasks.
Biosynthesis of lipids begins in the endoplasmic reticulum (ER), followed by distribution toward the plasma membrane and subsequent recycling back into the ER or catabolism in lysosomes, peroxisomes, and mitochondria.
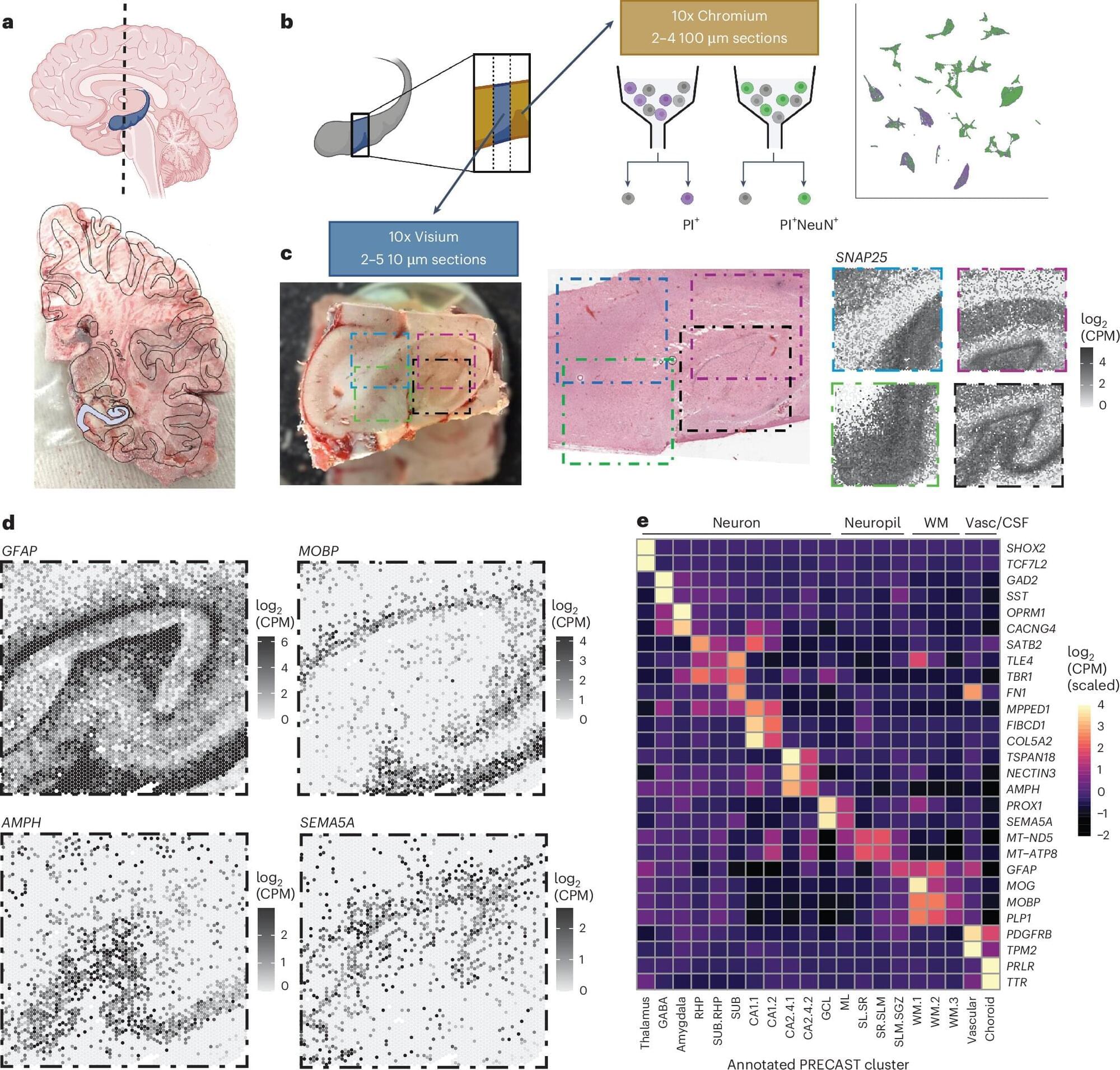
The hippocampus is an important brain region known to support various cognitive (i.e., mental) processes, including the encoding and retrieval of memories, learning, decision-making and the regulation of emotional states. While extensive research has tried to delineate the structure, functions and organization of the hippocampus, the cell types contained within it and their connections with other neurons have not yet been fully mapped out.
Over the past decades, available methods for studying cell subpopulations, the expressions of genes within them and their connectivity have become increasingly advanced. One of these methods, known as spatially resolved transcriptomics, works by measuring the expression of genes in cells while preserving their arrangement in space. Another called single-nucleus RNA-sequencing (snRNA-seq), allows scientists to examine RNA molecules inside individual cell nuclei to detect differences between them and categorize cells into different subtypes.
Researchers at Johns Hopkins Bloomberg School of Public Health, the Lieber Institute for Brain Development and Johns Hopkins School of Medicine recently used a combination of these two experimental techniques to examine cells in tissue extracted from the hippocampus. Their paper, published in Nature Neuroscience, introduces a comprehensive molecular atlas of the hippocampus that maps different cell subtypes and their organization.
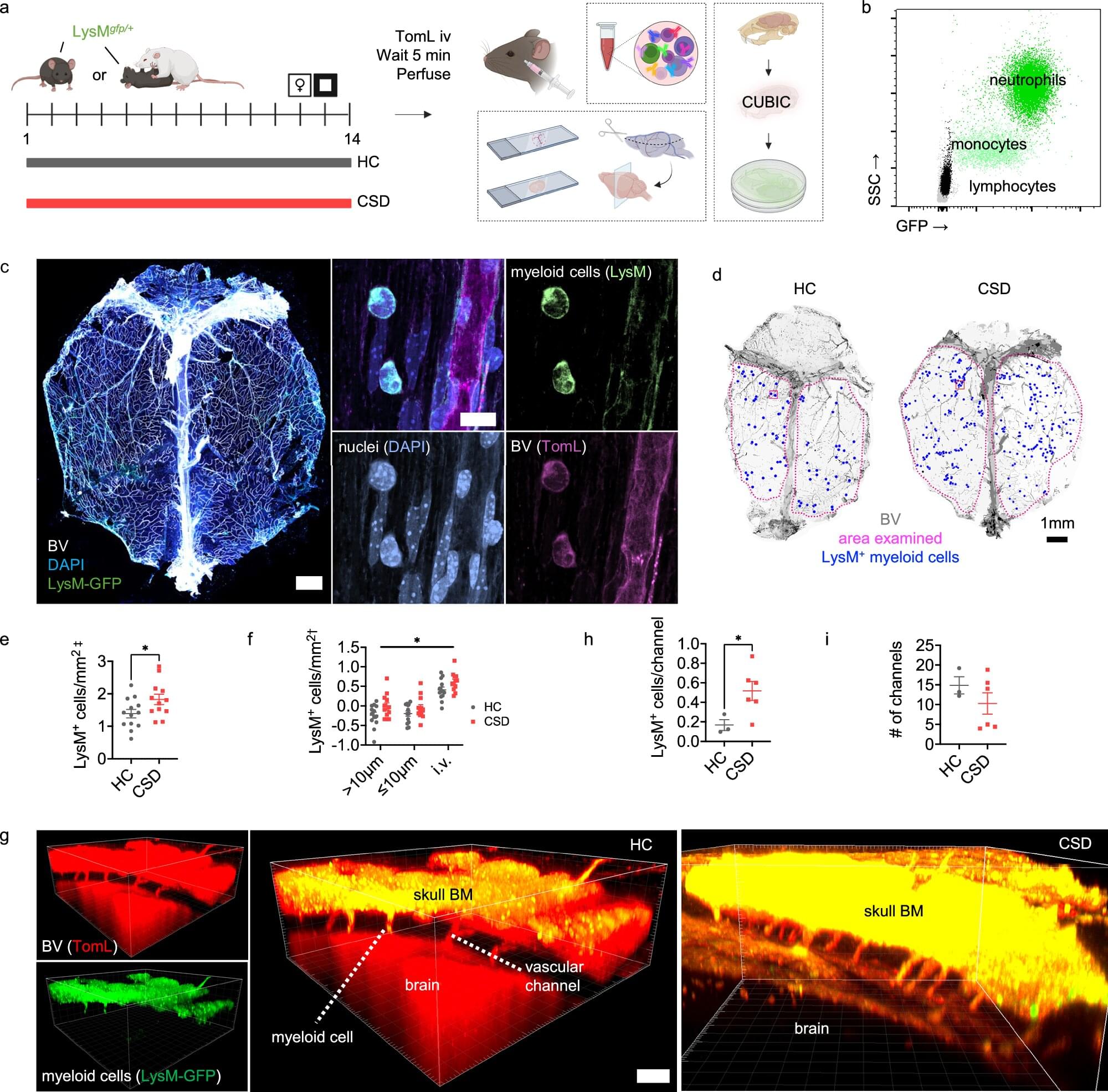
Immune cells released from bone marrow in the skull in response to chronic stress and adversity could play a key role in symptoms of depression and anxiety, say researchers.
The discovery—found in a study in mice—sheds light on the role that inflammation can play in mood disorders and could help in the search for new treatments, in particular for those individuals for whom current treatments are ineffective.
Around 1 billion people will be diagnosed with a mood disorder such as depression or anxiety at some point in their life. While there may be many underlying causes, chronic inflammation —when the body’s immune system stays active for a long time, even when there is no infection or injury to fight—has been linked to depression. This suggests that the immune system may play an important role in the development of mood disorders.
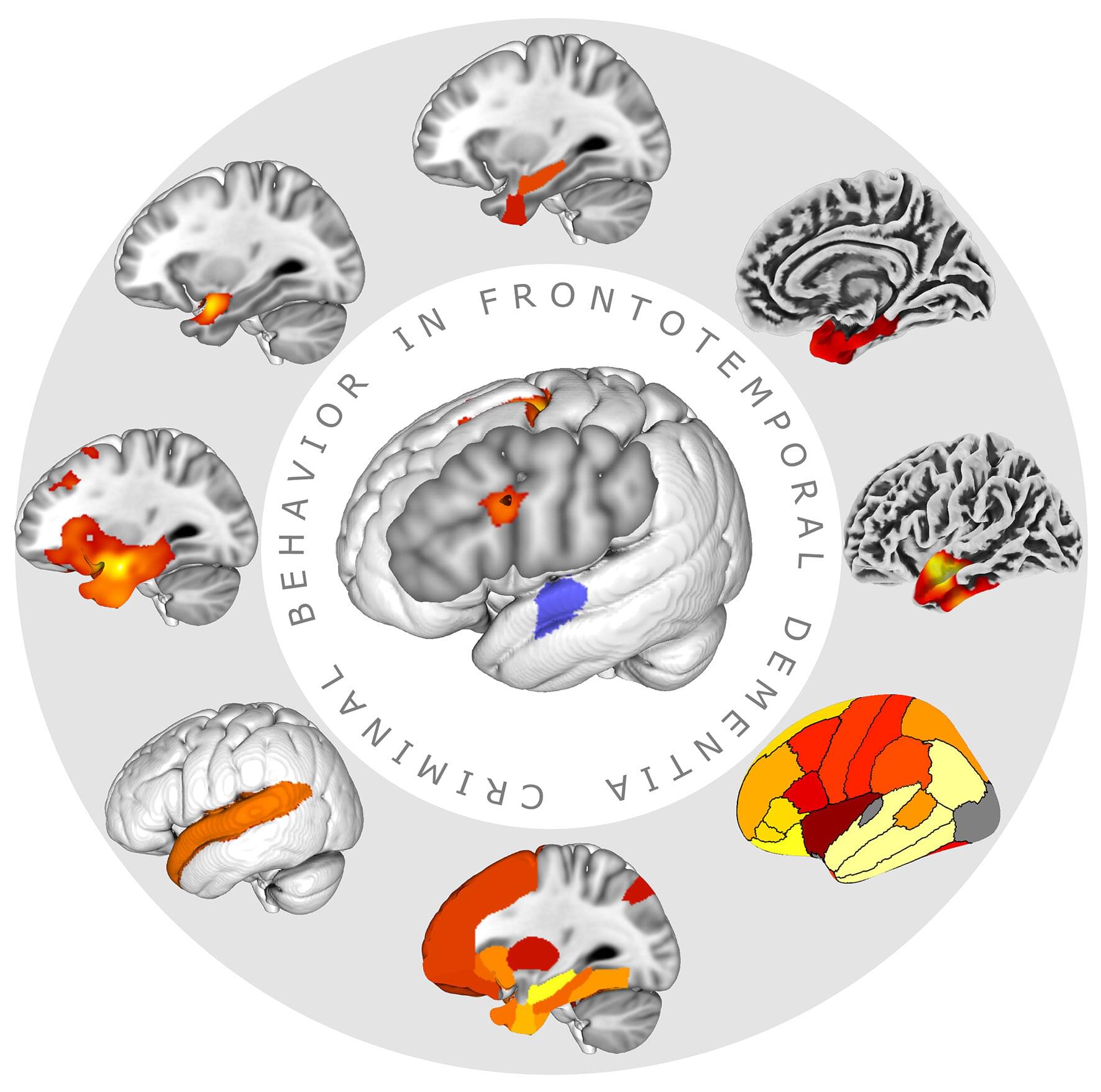
A suspected perpetrator who can barely remember his name, several traffic violations committed by a woman in her mid-fifties who is completely unreasonable and doesn’t understand her behavior—should such cases be brought before a court? And how does the state deal with people who commit acts of violence without meaning to?
Those questions come to mind if one hears those examples from everyday clinical praxis with persons suffering from dementia. Neurodegenerative diseases might affect several functions of the brain, ranging from memory in Alzheimer’s disease to behavior, such as in behavioral variant frontotemporal dementia, and to sensorimotor function in Parkinson’s disease.
One of the most interesting consequences of these alterations is the fact that persons affected by these diseases might develop criminal risk behavior like harassment, traffic violation, theft or even behavior causing harm to other people or animals, even as the first disease sign.

With the power to rewrite the genetic code underlying countless diseases, CRISPR holds immense promise to revolutionize medicine. But until scientists can deliver its gene-editing machinery safely and efficiently into relevant cells and tissues, that promise will remain out of reach.
Now, Northwestern University chemists have unveiled a new type of nanostructure that dramatically improves CRISPR delivery and potentially extends its scope of utility.
Called lipid nanoparticle spherical nucleic acids (LNP-SNAs), these tiny structures carry the full set of CRISPR editing tools—Cas9 enzymes, guide RNA and a DNA repair template—wrapped in a dense, protective shell of DNA. Not only does this DNA coating shield its cargo, but it also dictates which organs and tissues the LNP-SNAs travel to and makes it easier for them to enter cells.