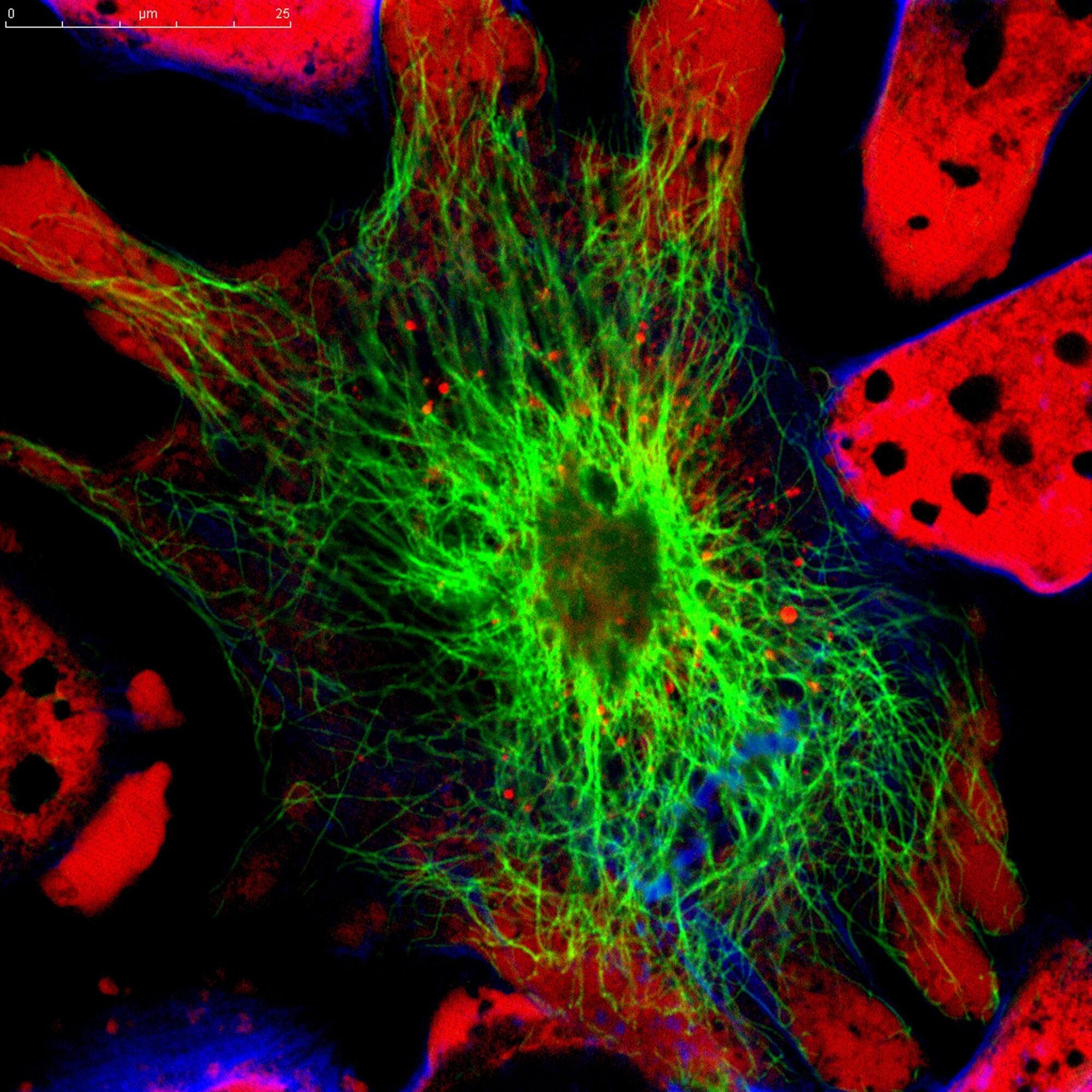
Scientists at the University of Nottingham have discovered surface patterns that can drastically reduce bacteria’s ability to multiply on plastics, which means that infections on medical devices, such as catheters, could be prevented.
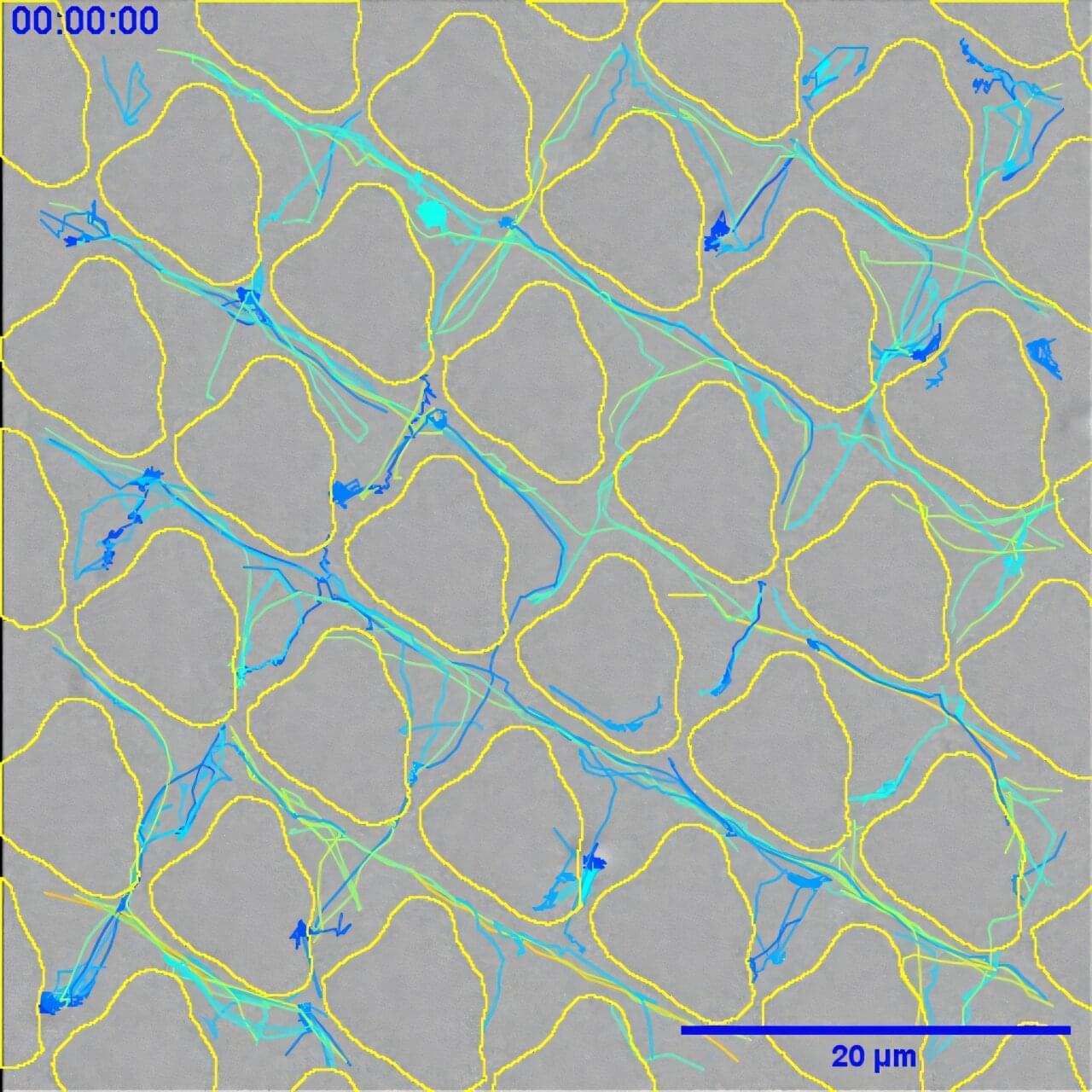
The findings of the study, which are published in Nature Communications, show that when bacterial cells encounter patterned grooves on a surface, they lose their ability to form biofilms.
Biofilms are surface-associated slime-cities which help protect the bacteria from the body’s natural defenses against infection. This, in turn, means the infection is effectively prevented before it can become fully established and would also positively activate the immune system to get rid of any individual bacteria that were there.