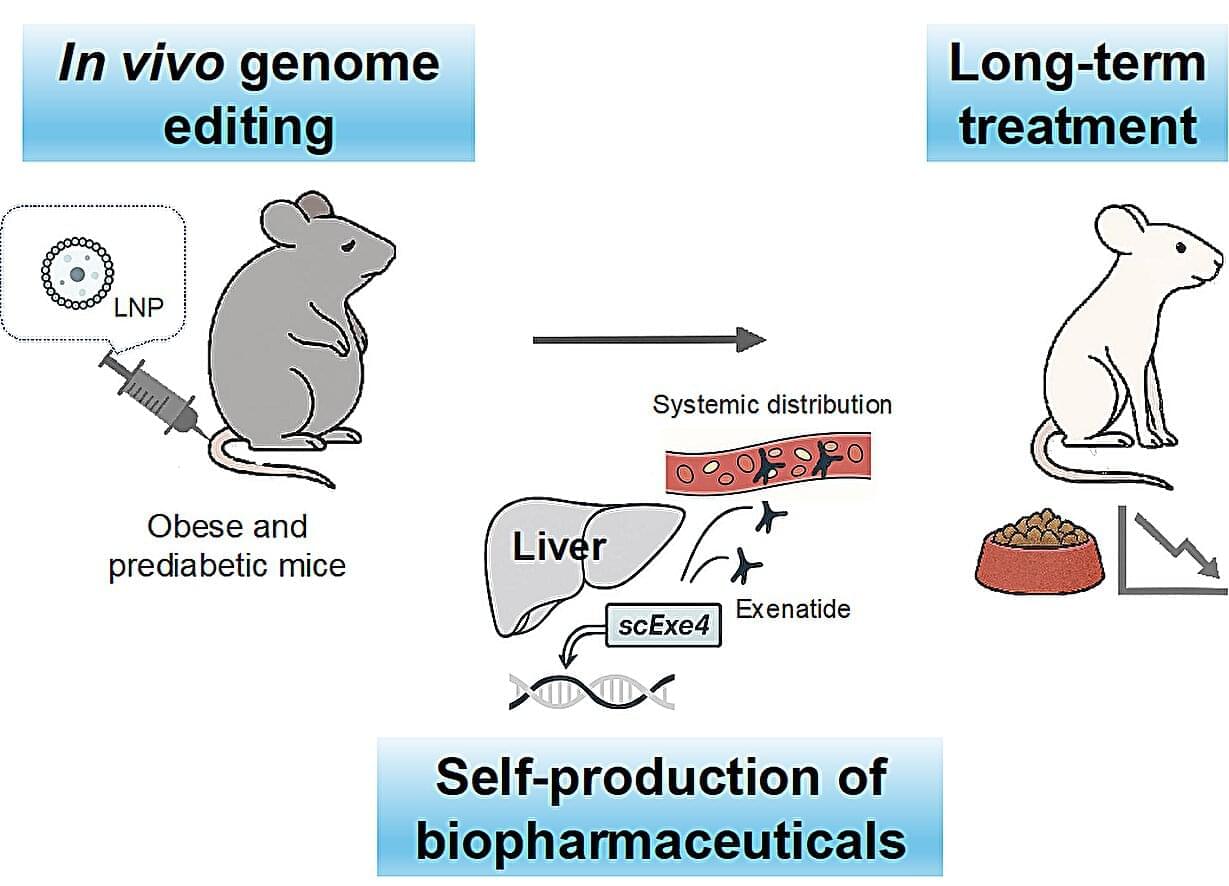
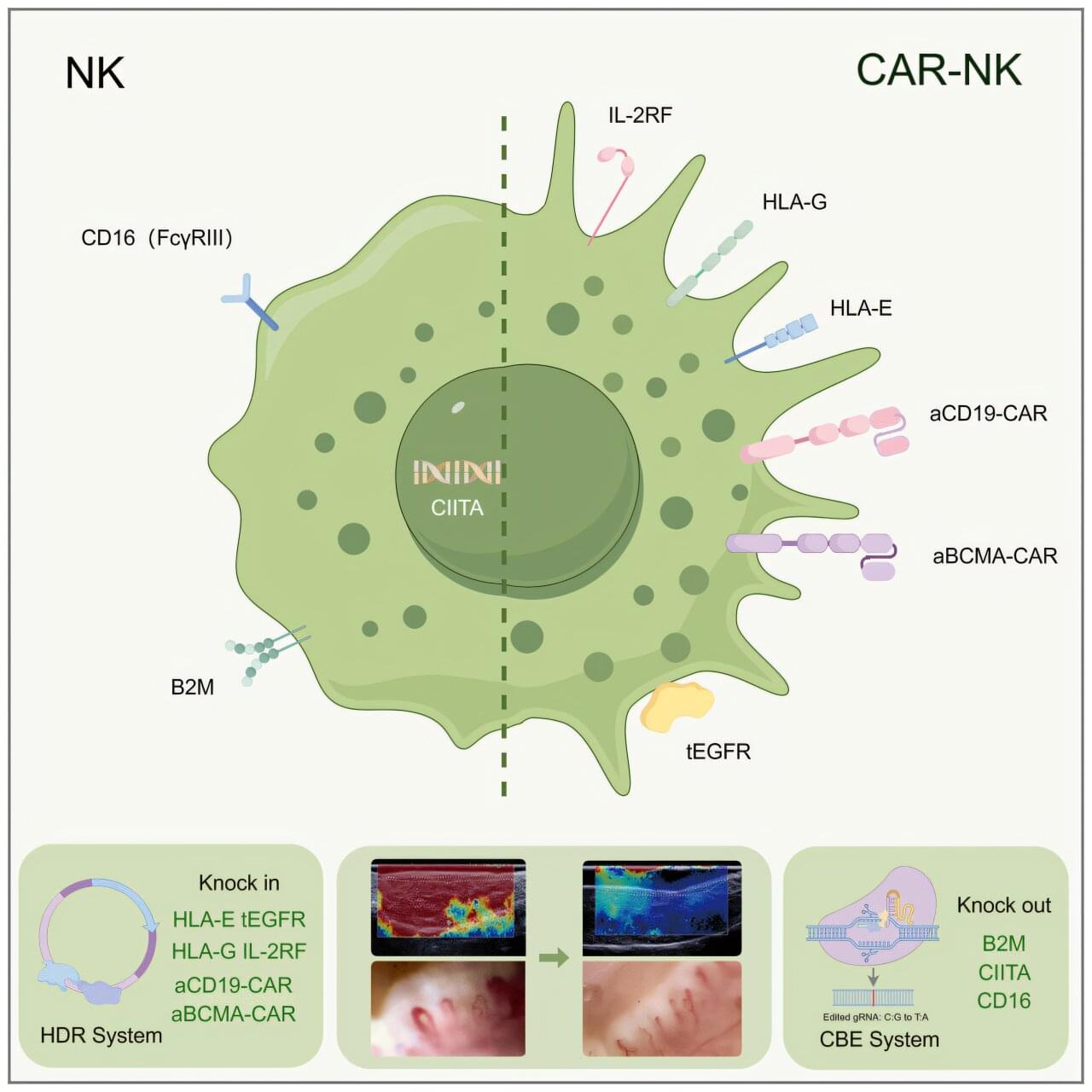
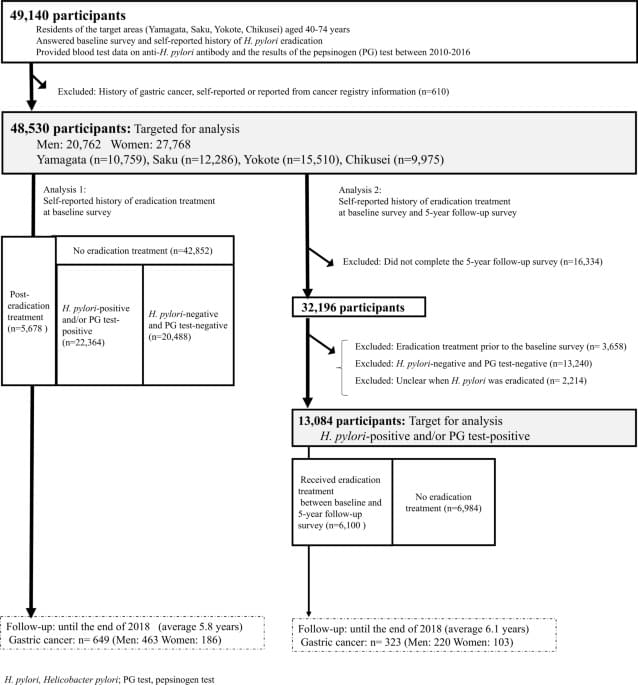
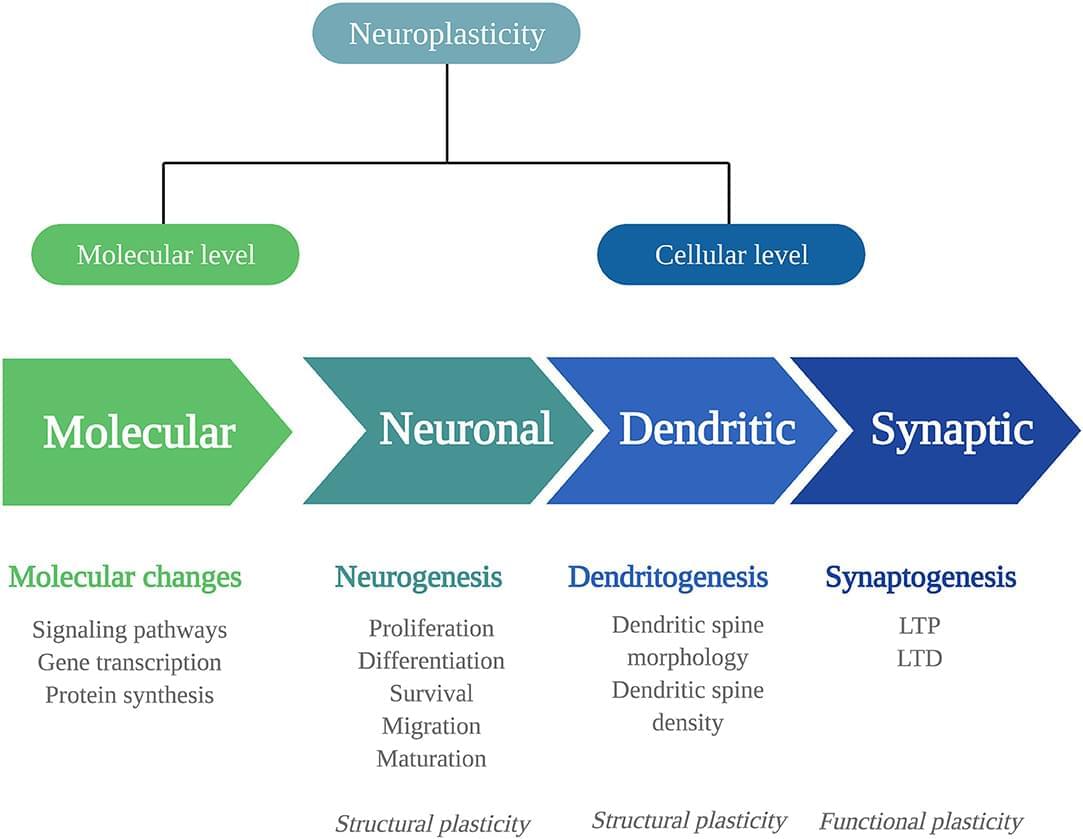
Even in moderation, consumption of ultra-processed foods is linked with measurable increases in risk for chronic diseases, according to research from the Institute for Health Metrics and Evaluation at the University of Washington. Processed meat, sugar-sweetened beverages (SSBs), and trans fatty acids (TFAs) were associated with an increased disease risk, such as type 2 diabetes, ischemic heart disease (IHD), and colorectal cancer.
Multiple previous studies have linked ultra-processed foods, particularly processed meats, sugar-sweetened beverages, and trans fatty acids, with elevated chronic disease risks. Estimates suggest that diets high in processed meat contributed to nearly 300,000 deaths worldwide in 2021, while diets rich in sugar-sweetened beverages and trans fats accounted for millions of disability-adjusted life years.
Processed meats preserved through smoking, curing or chemical additives often contain compounds such as N-nitroso agents, polycyclic aromatic hydrocarbons and heterocyclic amines—compounds implicated in tumor development.