3D printing drugs is driving the pharmaceutical industry towards personalized medicine. Let’s take a look at the most recent trends and developments.


Americans today spend more on pharmaceuticals per capita than anyone else in the world, and more than one in five say they have trouble affording their prescription drugs. But they might not know about the alternative pathways to medicine. VICE’s Hamilton Morris explores the world of clandestine chemists, DIY bio hackers, and grey markets to see if a more democratized medicinal future is indeed possible.
Check out VICE News for more: http://vicenews.com
Follow VICE News here:
Facebook: https://www.facebook.com/vicenews
Twitter: https://twitter.com/vicenews
Tumblr: http://vicenews.tumblr.com/
Instagram: http://instagram.com/vicenews
More videos from the VICE network: https://www.fb.com/vicevideo
#VICEonHBO

A computer virus forced a US maritime base offline for more than 30 hours, the country’s coast guard has revealed.
Ransomware interrupted cameras, door-access control systems and critical monitoring systems at the site.
The agency did not reveal the name or the location of the facility targeted by the attack.

Whenever someone refers me to a story with alarming facts that should surprise or outrage any thinking human, my spider-sense is activated. Does the story make sense? Is it plausible? If the message contains evidence of being repeated (or forwarded to more than two friends), then whatever is claimed is almost certain to be false.
If the subject is important to me—or if there is any chance that it might influence my view of the world, I check it at Snopes. The reputable web site confirms or debunks many urban legends and all sorts of viral web hype.
You never know what you might learn at Snopes. You can easily be lured into a rabbit hole, digging into the site beyond whatever prompted your visit in the first place.
Fact-checking can be fun! For example:

VITAMINS-Nutrients have long been described as healers-Life extending amino acids-chemicals… As has been done in the past holds true presently. There are trained professionals who for one reason or another attack the vitamin industry.
(Many claiming that vitamins do nothing and are washed from the body???)
How then did niacin extraction and synthesizing and being put into foods help end many sicknesses??? Yes Niacin is listed as a vitamin and a medicine…
So why is so much done and said against supplements??? Snake oil salesman and Big Pharma. I hate the term snake oil but it has been used against me and many others such as Doctor Aubrey de Grey…
SADLY There are those minds that have lied and harmed many down through the years by conartist-snake oil salesman… BUT For every bad product in nutrients there are 5 and more that are not so… WHAT SAY YE??? AEWR gerevivify.blogspot.com/
Jan. 2 (UPI) — While the vast majority of over-the-counter nutritional and herbal supplements are safe — unless they are consumed in large quantities — not all of them deliver on their promised benefits.
The great Elizabeth Parrish on ageing the most sinister disease on earth… I hate it when words are used to make aging sound like a normal sickness or a great sickness, Such as grandest??? Or most Important disease??? The decomposer disease that Woman-man has called natural aging all these years has been in reality a clandestine plague so complicated yet so easily seen by the naked eye if certain scholars-textbooks do not get in the way…
Aging is The Eukaryotic Cellular pandemic plague AEWR has named the Senesonic-Sensonic plague. A disease that causes all of our cells to age nearly at the same rate causing our cells to have to regenerate the day long or the body drops.
WHEN without the Plague our cells individually would live decades instead of mere hours. Causing a bodily effect of Super Longevity-truly Immortality living into millennia once the plague is ended. SO IT IS NOT Liz’s fault, {I met her at RAADFEST 2018 she is a very special mind} but it is the pioneers of biology’s fault that the Human being has died needlessly for now centuries.
Aging could have been cured 200 and more years ago… I search for strong willed men and Women to now join AEWR as investors-partners in agings now end. We have found the causes and yes a very expensive cure we named the Gerevivify Algorithm… gerevivify.blogspot.com/ WE HAVE MUCH TO DO… r.p.berry & AEWR.
The English philosopher Thomas Hobbes correctly identified the historical state of humans when he said “Life in the state of nature is nasty, brutish and short.” For most of history the average human rarely lived beyond 35 years, but today the average Briton lives beyond 85 years. In this video, Liz argues that this luxury of time helps us realise that we must live with the consequences of our actions — which facilitates caring about our environment and each other. Liz’s organisation, BioViva, intend to accelerate this trend by using advanced medicine to increase health and longevity beyond 100 years for everyone.
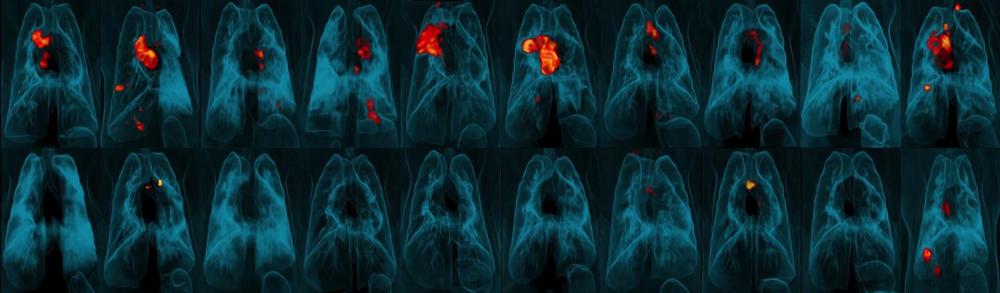
Worldwide, more people die from tuberculosis (TB) than any other infectious disease, even though the vast majority were vaccinated. The vaccine just isn’t that reliable. But a new Nature study finds that simply changing the way the vaccine is administered could dramatically boost its protective power.
Researchers at the University of Pittsburgh School of Medicine and the National Institute of Allergy and Infectious Diseases (NIAID) discovered that intravenous TB vaccination is highly protective against the infection in monkeys, compared to the standard injection directly into the skin, which offers minimal protection.
“The effects are amazing,” said senior author JoAnne Flynn, Ph.D., professor of microbiology and molecular genetics at the Pitt Center for Vaccine Research. “When we compared the lungs of animals given the vaccine intravenously versus the standard route, we saw a 100,000-fold reduction in bacterial burden. Nine out of 10 animals showed no inflammation in their lungs.”
My mission is to drastically improve your life by helping you break bad habits, build and keep new healthy habits to make you the best version of yourself.
- Please consider donating: https://paypal.me/BrentNally or my Bitcoin Cash (BCH) address: qr9gcfv92pzwfwa5hj9sqk3ptcnr5jss2g78n7w6f2
Follow Brent on social media:
- Instagram: https://instagram.com/brent.nally/
- Facebook: https://facebook.com/brent.nally
- LinkedIn: https://linkedin.com/in/brentnally
- Twitter: https://twitter.com/BrentNally
- Patreon: https://patreon.com/user?u=9451534
This video is My 1 year Mesenchymal Stem Cells Update from my December 20, 2018 treatments at Dream Body Clinic in Nuevo Vallarta, Mexico. Dream Body Clinic works with CryoVida (www.cryovida.com.mx) to receive the MSCs. CryoVida is the only lab in Mexico with 3 laboratory certifications for stem cell cultivation and research.
The MSCs are extracted from the Wharton’s Jelly of the umbilical cords of young and healthy (disease free) mothers in CryoVida’s fertility clinic in Guadalajara, Mexico which is about a 5 hour drive from the Dream Body Clinic in Nuevo Vallarta where I received the IV infusion and local injections of the MSCs. The MSCs are immediately refrigerated after extraction and stay refrigerated until injection &/or infusion. The MSCs must be injected within 4 days from birth or they must be discarded. CryoVida has been doing this since 2002. Many other companies all over the world have used umbilical cord derived MSCs for various treatments and this treatment is rapidly growing in popularity. I wouldn’t put these MSCs in my body if I didn’t do extensive research to verify that it’s safe. Everyone should do their own research to determine if they feel it’s safe enough to put in their body.