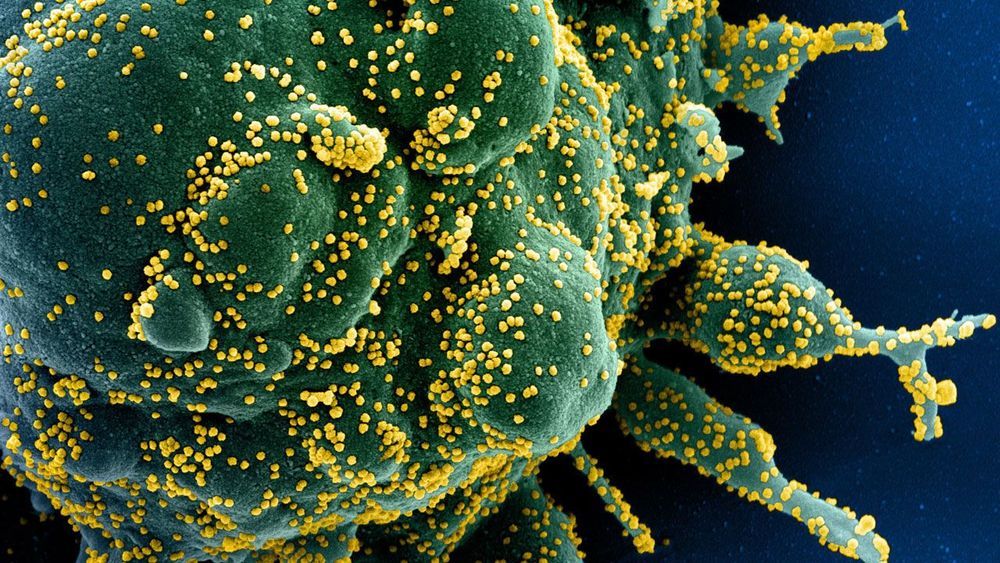
New findings suggest past infections may offer some protection against the novel coronavirus.


People, bicycles, cars or road, sky, grass: Which pixels of an image represent distinct foreground persons or objects in front of a self-driving car, and which pixels represent background classes?
This task, known as panoptic segmentation, is a fundamental problem that has applications in numerous fields such as self-driving cars, robotics, augmented reality and even in biomedical image analysis.
At the Department of Computer Science at the University of Freiburg Dr. Abhinav Valada, Assistant Professor for Robot Learning and member of BrainLinks-BrainTools focuses on this research question. Valada and his team have developed the state-of-the-art “EfficientPS” artificial intelligence (AI) model that enables coherent recognition of visual scenes more quickly and effectively.
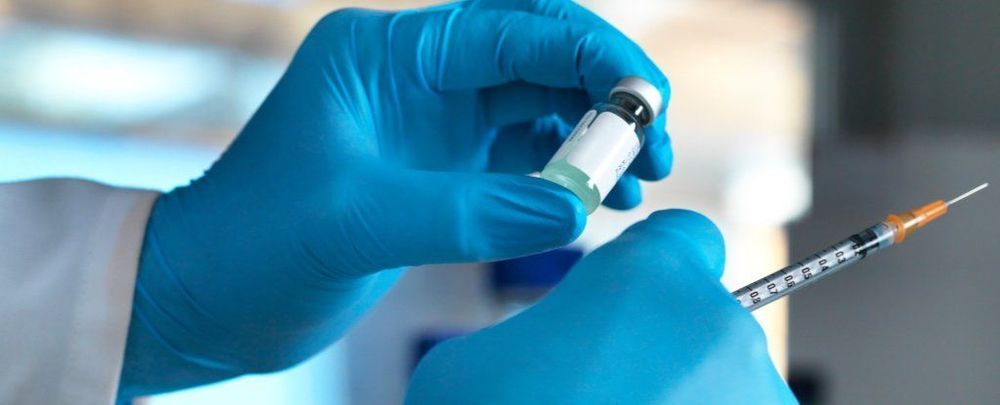
MONDAY, May 11, 2020 (HealthDay News) — An experimental vaccine seems to give monkeys extended protection from an HIV-like infection — by “waking up” an arm of the immune system that vaccines normally do not.
Experts cautioned that animal research often does not pan out in humans. The decades of work toward an HIV vaccine has been a clear example. But, researchers said, this vaccine works differently, targeting two “arms” of the immune system.
And they think the work potentially has broader lessons for vaccines being developed for other viruses, including SARS-CoV-2, the virus that causes COVID-19.
There may be hope yet for a universal flu vaccine — one powerful dose of immunisation that can provide long-lasting protection for multiple influenza strains, all in a single shot.
A discovery like that would be a holy grail for public health, and after more than a decade of careful research, a specific version called FLU-v is now moving into the last rounds of clinical testing.
So far, researchers say the results have been “very encouraging”, and the vaccine has successfully passed phase I and phase II clinical trials. Although trials in these phases are limited to assessing the safety of the vaccine, there’s also evidence it might be effective.

O,.o!
An international consortium found a remarkable global spread of strains of a multi-resistant bacterium that can cause severe infections—Stenotrophomonas maltophilia. The study, published under the supervision of the Research Center Borstel Leibniz Lung Center (FZB), provides for the first time a systematic understanding of the global phylogeny of S. maltophilia strains and shows ways to efficiently monitor the pathogen using a genomic classification system. DZIF scientists from Lübeck, Borstel and Braunschweig are involved in the study.
S. maltophilia strains occur in several natural and human associated ecosystems. The bacterium was long regarded as relatively unproblematic but is now considered to be one of the most feared hospital pathogens, as it frequently causes infections and is resistant to a number of antibiotics. This can be particularly dangerous for immune-compromised patients or for patients with underlying inflammatory lung diseases such as cystic fibrosis. Although almost any organ can be affected, infections of the respiratory tract, bacteraemia or catheter-related infections of the bloodstream are the most common. In view of the increasing importance of this pathogen and the often-severe clinical consequences of an infection, knowledge about the virulence factors and about the local and global transmission of S. maltophilia bacteria is urgently needed.
Scientists from a total of eight countries initially established a genotyping method that enables the standardised analysis of the different genomes of S. maltophilia strains. The DZIF teams around Prof. Stefan Niemann (FZB), Prof. Jan Rupp, (Clinic of Infectiology and Microbiology, Campus Lübeck) and Prof. Ulrich Nübel from the Leibniz Institute DSMZ (German Collection of Microorganisms and Cell Cultures GmbH) in Braunschweig were involved.
What it is, where it comes from, how it hurts us, and how we fight it.
By
Ira Pastor, ideaXme life sciences ambassador, interviews Dr. Mark Wolff, Morton Amsterdam Dean, and Professor, Division of Restorative Dentistry, at the University of Pennsylvania, School of Dental Medicine.
Ira Pastor Comments:
So as frequent listeners of the ideaXme show know, we spend a lot of time talking about the theme of “healthy aging”, and the reality that aging, and related biological changes associated with aging, occur across all of the body’s cells, tissues, and organs, and these changes affect all structure and function of the body, including the teeth and gums, and as such, oral health is the focus of our show today.
The underlying processes of biological aging can dramatically affect oral health and many of the specific changes that occur over time in our bodies as we age (such as cells renewing at a slower rate, tissues become thinner and less elastic, bones become less dense and strong, our immune system become weaker, such that infection can occur more quickly and wound healing takes longer), can have major impact in the oral cavity (affecting tissue and bone in the mouth), as well as trickle down effects on the rest of the body.
Oral health problems in older adults include, but are not limited to: untreated tooth decay, gum disease, tooth loss, oral cancer, as well as exacerbated chronic disease associated with various co-morbid conditions and physiologic changes associated with aging (e.g., hypertension, diabetes mellitus).
Dr. Mark Wolff:



The Plasma Protein Therapeutics Association (PPTA) and its member companies are closely monitoring the rapidly changing information landscape regarding the spread of Novel Coronavirus 2019 (COVID-19) and wish to be a resource for patients, plasma donors, policymakers, and other stakeholders during this pandemic.
Plasma donors save lives! Your donations are even more essential during the current COVID-19 pandemic to ensure patients with rare diseases can keep receiving their lifesaving treatments. Authorities in the U.S. and in the EU have published guidance documents recognizing the essential nature of plasma donation. Please click here to find a plasma donation center near you.