A major medical breakthrough is giving new hope to those with Parkinson’s disease. Stem cell transplants into the brain are showing impressive results, leading to long-term improvements in motor symptoms for the first patients treated… Read more



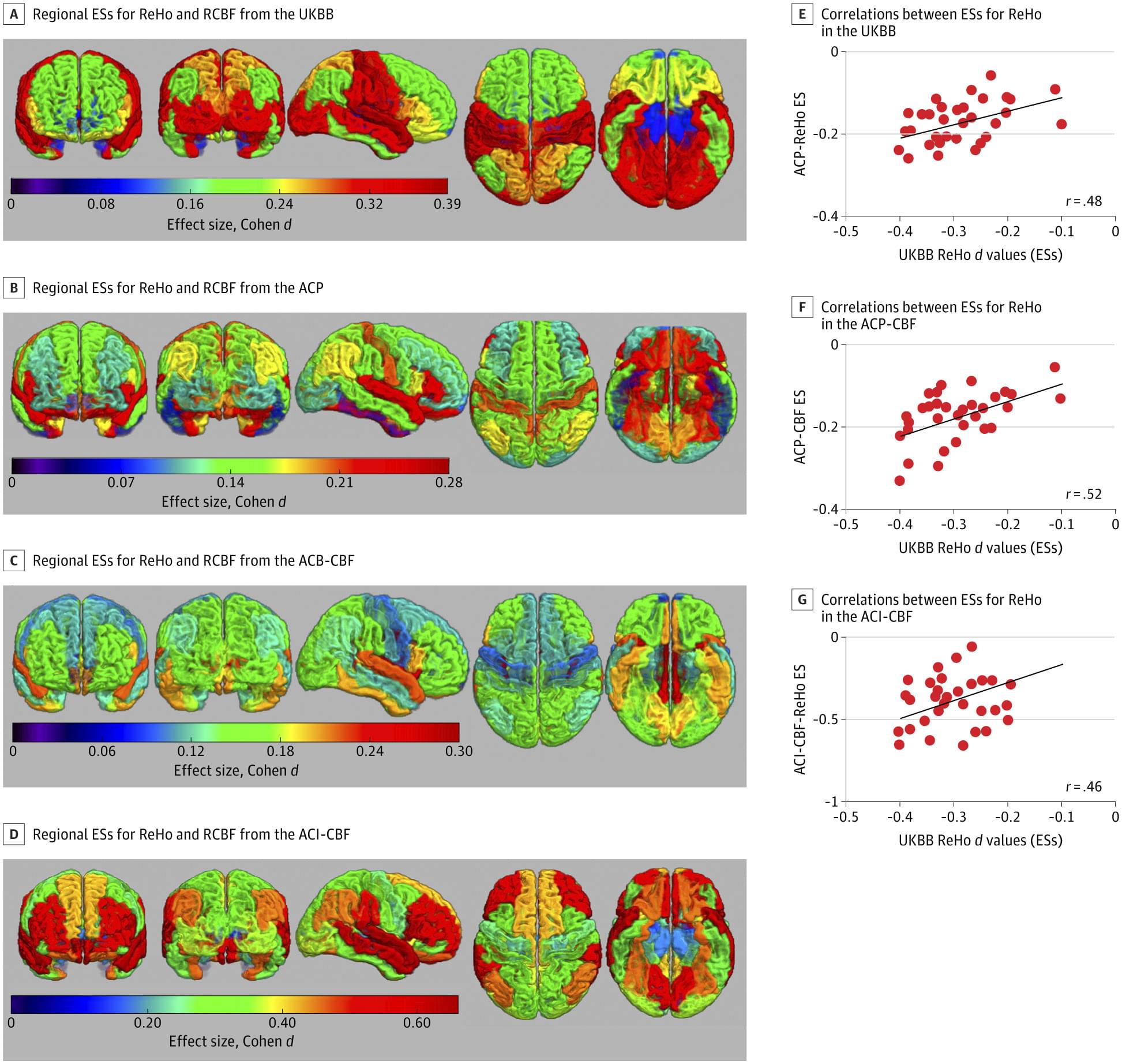
The study provides critical new insights into the African Humid Period, a time between 14,500 and 5,000 years ago when the Sahara desert was a green savanna, rich in water bodies that facilitated human habitation and the spread of pastoralism. Later aridification turned this region into the world’s largest desert. Due to the extreme aridity of the region today, DNA preservation is poor, making this pioneering ancient DNA study all the more significant.
Genomic analyses reveal that the ancestry of the Takarkori rock shelter individuals primarily derives from a North African lineage that diverged from sub-Saharan African populations at about the same time as the modern human lineages that spread outside of Africa around 50,000 years ago. The newly described lineage remained isolated, revealing deep genetic continuity in North Africa during the late Ice Age. While this lineage no longer exists in unadmixed form, this ancestry is still a central genetic component of present-day North African people, highlighting their unique heritage.
An international team led by researchers from the Max Planck Institute for Evolutionary Anthropology in Leipzig, Germany, has sequenced the first ancient genomes from the so-called Green Sahara, a period when the largest desert in the world temporarily turned into a humid savanna-like environment. By analyzing the DNA of two 7,000-year-old naturally mummified individuals excavated in the Takarkori rock shelter in southwestern Libya by the Archaeological Mission in the Sahara, Sapienza University of Rome, the team showed that they belonged to a long-isolated and now extinct North African human lineage. This group of cattle pastoralists has only a minor genetic component of non-African ancestry, suggesting that animal husbandry may have spread into the Green Sahara through cultural exchange rather than large-scale migrations.
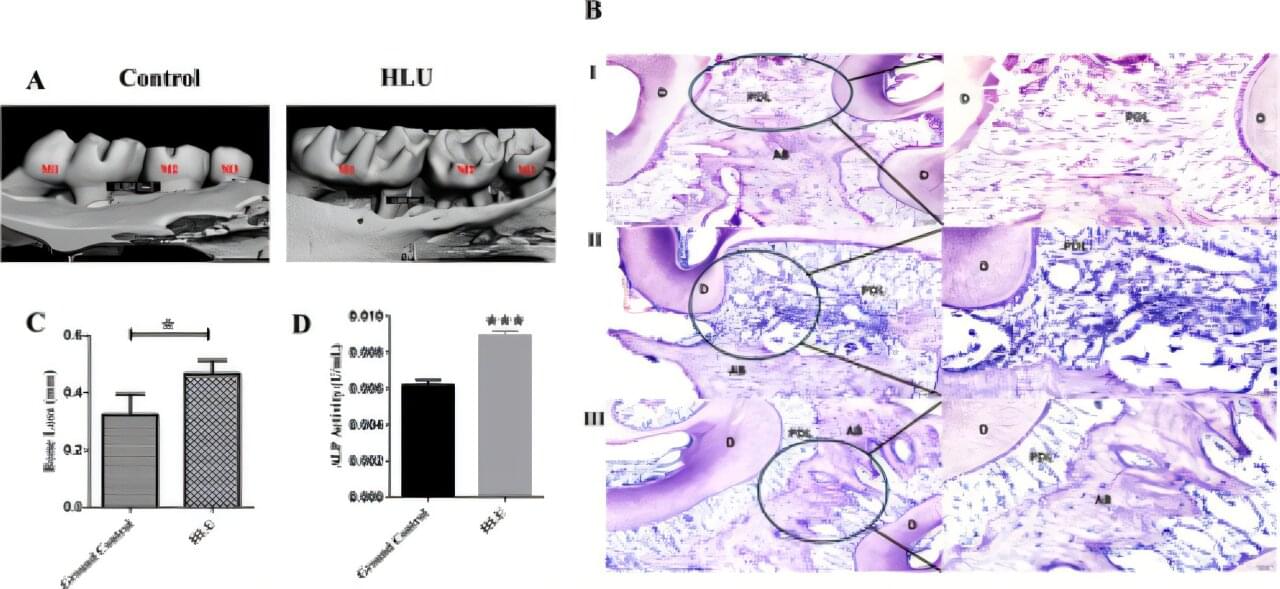
Living in zero gravity can lead to periodontitis, a common and serious condition where the gums become inflamed and the bone that supports teeth starts to break down, eventually leading to tooth loss, scientists reveal in a new study.
The scientists confirm their findings in a study published in the Journal of Periodontal Research, in which they try to understand how simulated microgravity —the near-weightless environment astronauts experience in space—might influence the development and severity of periodontitis.
The researchers carried out their experiment in a lab in which mice were used to test the impact of periodontitis in microgravity conditions and on Earth. To simulate this, they used a special model where mice were placed in a position that mimics the effects of microgravity, and then gum disease was induced.
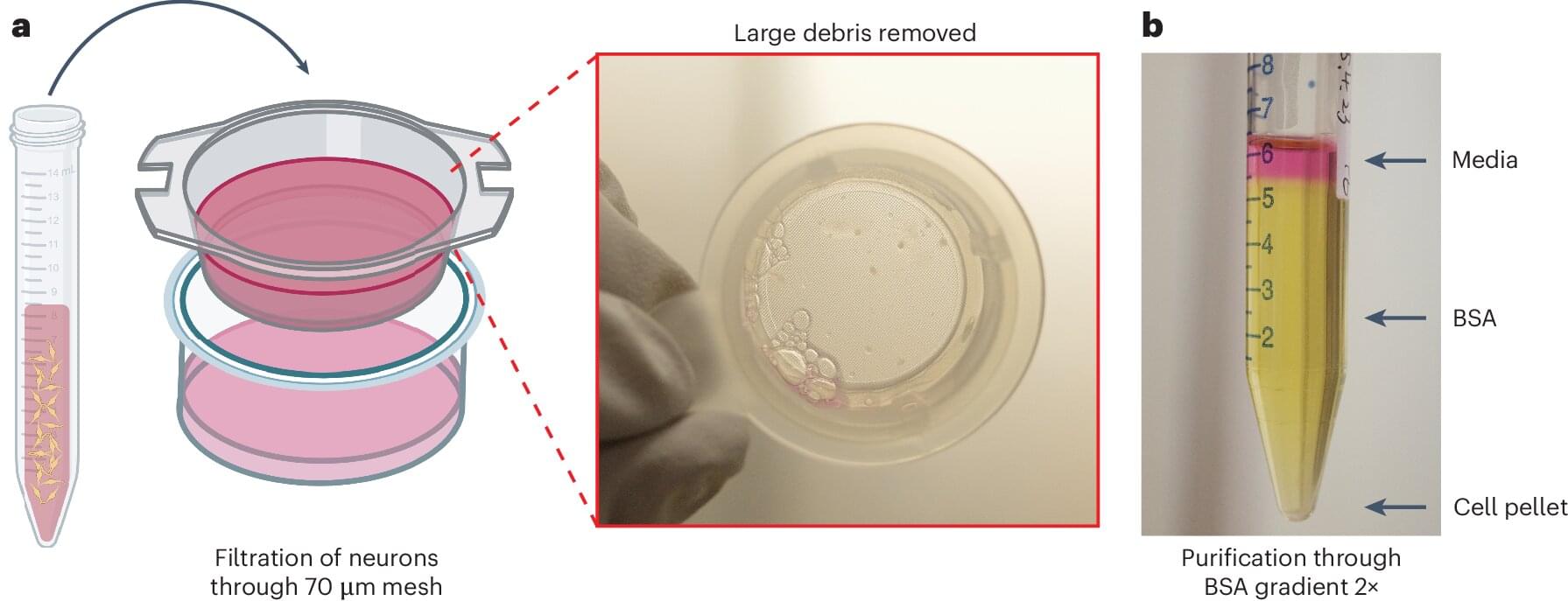
Understanding the electrical activity of neurons is key to unlocking insights into neurological diseases. Yale researchers have unveiled a high-throughput automated method that captures the electrical activity of large numbers of neurons simultaneously and without bias.
This cutting-edge approach provides a powerful “functional fingerprint” of neuron populations in their natural state, opening new doors to understanding and treating neurological diseases. The work was published June 13 in Nature Protocols.
The patch-clamp technique has long been a gold standard for studying the electrical activity of neurons, the fundamental units of the nervous system. However, the manual execution of this approach is slow and labor-intensive. Recent advances in robotic patch-clamp technologies have improved speed and efficiency, but they are limited to artificially grown neurons rather than neurons in their native unmanipulated state.
A newly discovered mechanism that leads to liver dysfunction may be a key factor in type 2 diabetes and other metabolic disorders in individuals with obesity, according to a new study led by Harvard T.H. Chan School of Public Health.
The dysfunction identified—dysregulated hepatic coenzyme Q metabolism—leads to excessive reactive oxygen species (ROS) produced by mitochondria at a single specific site in an enzyme called complex I. The researchers say the discovery offers a potential path for new, precise treatments for metabolic diseases.
“Our findings provide the first step toward solving a complex problem in the field of metabolic disease research that has stood for three decades,” said corresponding author Gökhan S. Hotamisligil, James Stevens Simmons Professor of Genetics and Metabolism.

Wastewater generated by animal farms poses a significant environmental risk, as it can pollute soil and groundwater and can be hazardous to human health. However, animal farm wastewater also contains carbon and many other nutrients. What if we could extract the carbon and nutrients and then release treated water back into the environment?
That’s the future envisioned by Prathap Parameswaran, an associate professor at Kansas State University who researches how to use environmental biotechnology platforms for biological wastewater treatment and sustainable resource recovery.
ABPDU and Kansas State University researchers make progress on extracting useful products from animal farm wastewater.
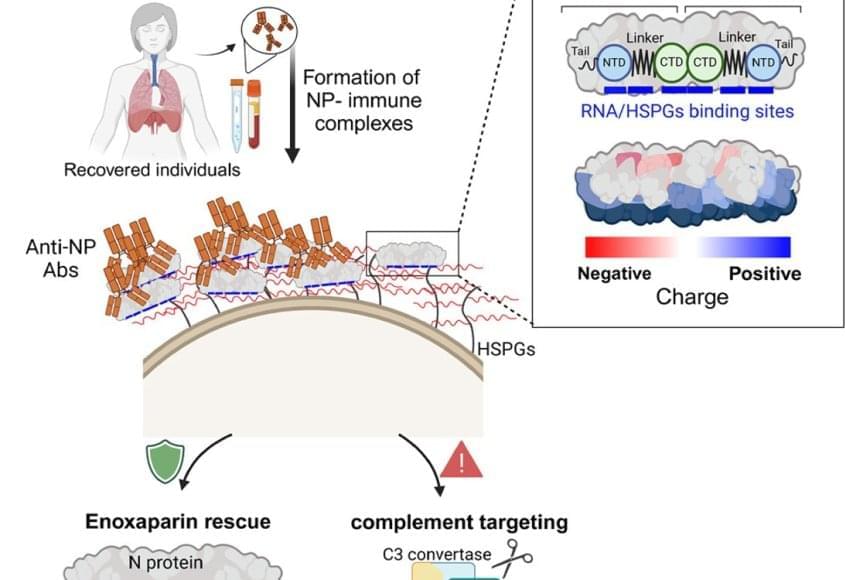
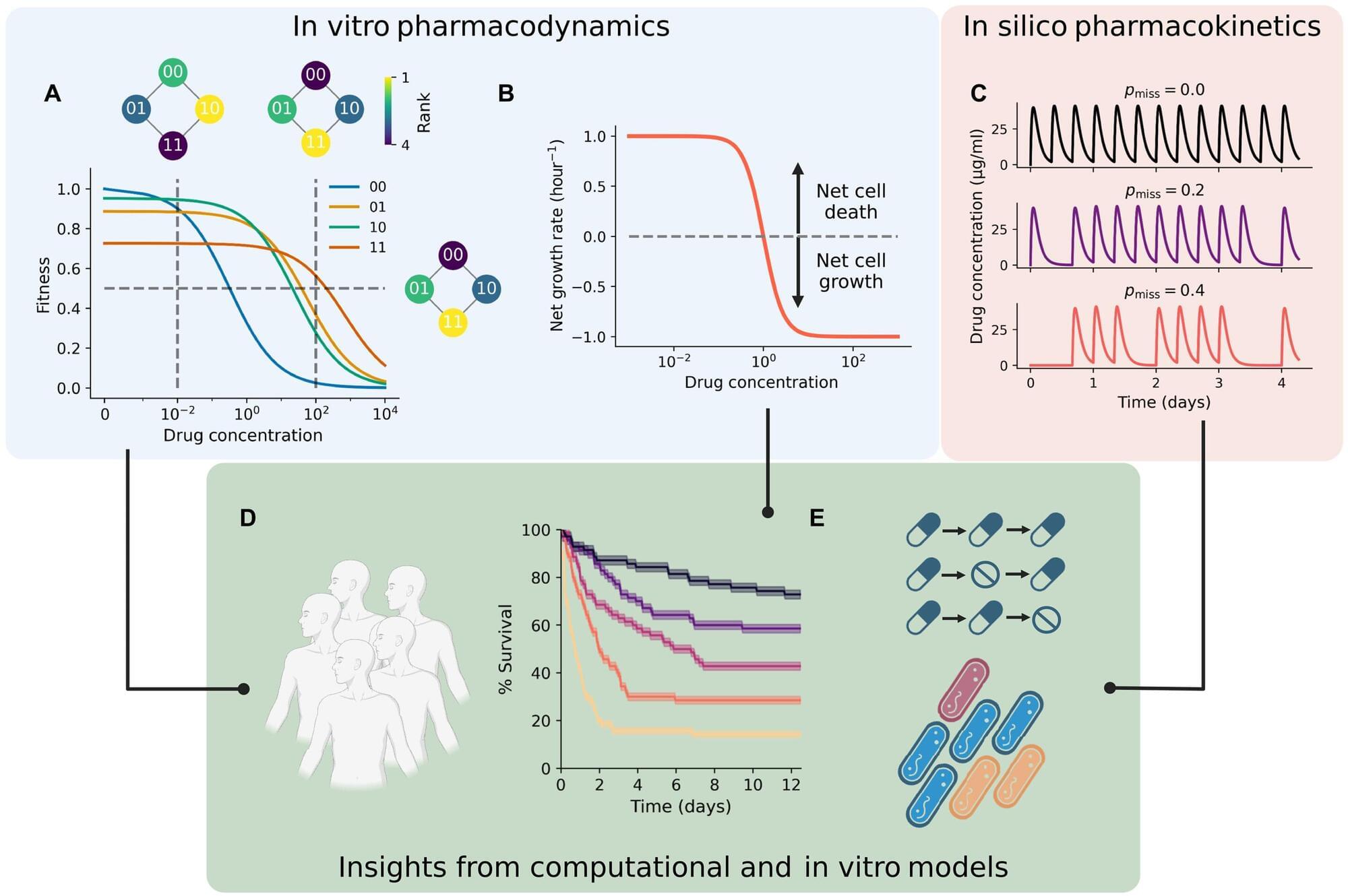
Cleveland Clinic researchers are working to improve the way we use evolutionary modeling to understand drug resistance. The study, published in Science Advances, uses a new type of evolutionary model called a “fitness seascape” to incorporate a patient’s dosage schedule into models that predict whether an infection will develop antibiotic resistance, and has found that inconsistent timing and missing early doses can lead to treatment failure.
A team led by Jacob Scott, MD, DPhil., including study first author Eshan King, an MD/Ph. D. student at Case Western Reserve University School of Medicine, is refining models that determine recommended antibiotic doses by incorporating bacterial evolutionary dynamics.
“With the rise of ‘superbugs,’ or antibiotic-resistant bacterial infections, the world is reaching a crisis point,” says Dr. Scott, the study’s senior author. “We’ve already seen from MRSA what can happen if a bacterium becomes antibiotic-resistant. We need to address the problem before it impacts our ability to use antibiotics in more routine aspects of medical care, like surgery or childbirth.”