Australian researchers have made a major breakthrough in HIV research by repurposing the same mRNA delivery system used in COVID-19 vaccines, not to prevent infection, but as a potential strategy to find a cure.
Nearly 40 million people live with HIV worldwide. While antiretroviral therapy can suppress the virus to undetectable levels, it cannot eliminate it. HIV has a unique ability to hide in a type of white blood cells, resting CD4+ T cells, ready to re-emerge if treatment is stopped. This HIV “reservoir” has long been one of the greatest challenges in the search for a cure.
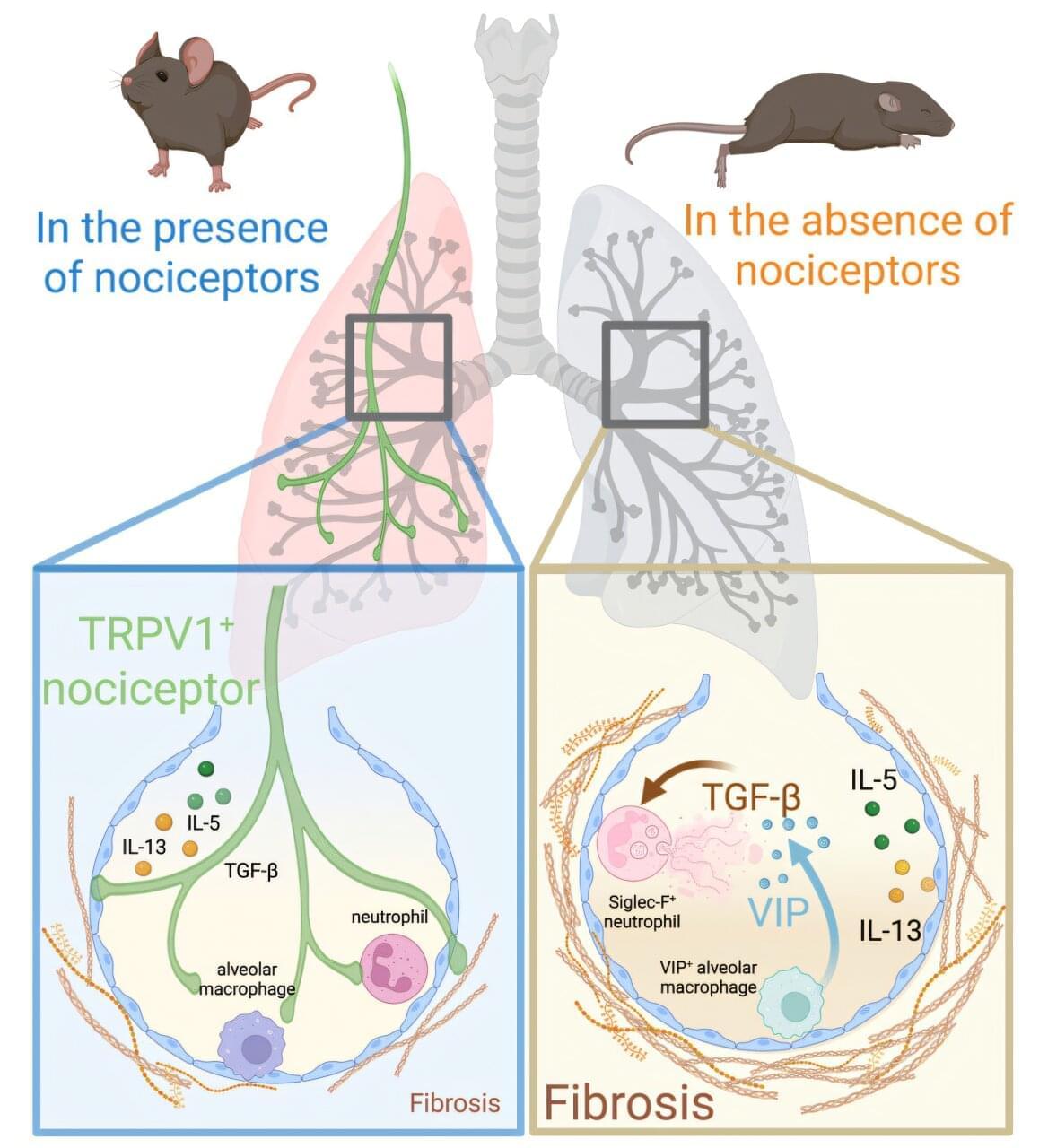
Using the same technology behind mRNA COVID-19 vaccines, researchers, led by the Peter Doherty Institute for Infection and Immunity (Doherty Institute), have discovered a new way to deliver mRNA to the elusive HIV reservoir and coax HIV out of hiding. In a laboratory-based study published in Nature Communications, the team packaged mRNA inside an entirely novel microscopic fat-like bubbles, known as lipid nanoparticles, and successfully transported it into HIV-infected cells, where it prompted the cells to expose the dormant virus.
Researchers from Professor Sharon Lewin’s laboratory at the Doherty Institute have made a major breakthrough in HIV research by repurposing the same mRNA delivery system used in COVID-19 vaccines, not to prevent infection, but as a potential strategy to find a cure.