Using this approach, researchers can map how light spreads in opaque environments.


Depression is a difficult illness. Not only does it make you feel like crap, but like so many primarily mental illnesses, it also comes with a bucketful of misinformation and misconceptions surrounding it. Even medical specialists, whom you’d expect to be the authorities on the matter, are stumped by some aspects of the disease – the truth is, while humanity may be more informed than ever on matters of the brain, we still really don’t know what’s going on inside of it when it glitches like this.
But that may soon change. Researchers based at Baylor College of Medicine in Houston, Texas, claim to have developed what they call a “mood decoder” – a way of reading people’s emotional state just from looking at brain activity.
“This is the first demonstration of successful and consistent mood decoding of humans in these brain regions,” Baylor College neurosurgeon and project lead Sameer Sheth told MIT Technology Review. And the best part? The team have also found a way to stimulate a positive mood in patients’ brains.
Anatomical decision-making by cellular collectives: Bioelectrical pattern memories, regeneration, and synthetic living organisms.
A key question for basic biology and regenerative medicine concerns the way in which evolution exploits physics toward adaptive form and function. While genomes specify the molecular hardware of cells, what algorithms enable cellular collectives to reliably build specific, complex, target morphologies? Our lab studies the way in which all cells, not just neurons, communicate as electrical networks that enable scaling of single-cell properties into collective intelligences that solve problems in anatomical feature space. By learning to read, interpret, and write bioelectrical information in vivo, we have identified some novel controls of growth and form that enable incredible plasticity and robustness in anatomical homeostasis. In this talk, I will describe the fundamental knowledge gaps with respect to anatomical plasticity and pattern control beyond emergence, and discuss our efforts to understand large-scale morphological control circuits. I will show examples in embryogenesis, regeneration, cancer, and synthetic living machines. I will also discuss the implications of this work for not only regenerative medicine, but also for fundamental understanding of the origin of bodyplans and the relationship between genomes and functional anatomy.
How do our bodies know what to become?
There are no instructions in our genes that code for the exact 3D structure of our bodies. There’s no tiny human contained in our DNA. So, what powers the transformation of the first cell in the embryo to a full-blown organism?
Dr Michael Levin is attacking this problem and, in the process of answering it, his lab is uncovering an entirely new way of looking at biology.
== What we talk about ==
0:04 — Introduction.
1:20 — You were a software engineer. How did you get interested in biology?
6:50 — Can bacteria exhibit intelligent behavior?
7:46 — How do organisms take their final shape?
22:51 — How do cells in our body know when to stop multiplying?
27:49 — Analogs of software and hardware in developmental biology.
34:20 — Where are the body plans stored in complex organisms like ours?
43:33 — What post-DNA paradigms are important in biology?
48:20 — What is regenerative medicine?
50:20 — How far have we progressed in regenerative medicine?
52:52 — Xenobots: world’s first synthetic organisms.
1:00:12 — How to program Xenobots.
1:05:13 — How do you handle the ethical dilemma while you are working with conscious organisms?
1:10:22 — How do you enable the scientific creativity in your lab and amongst your students? And is it a teachable skill?
== About the guest ==
Michael Levin is a Distinguished Professor in the Biology department at Tufts and serves as director of the Allen Discovery Center. He holds a PhD in biology from the Harvard University. At Tufts, his research group is interested in figuring out how our bodies know what to become.
He believes that what guides our body plans is bio-electric communication between different units. Our bodies take shape the way they have because each of our subunits — cells, tissues, organs — collectively decides it to be that way.
Gate’s team of scientists observed genetic changes in the CSF immune cells in older healthy individuals that made the cells appear more activated and inflamed with advanced age.
“The immune cells appear to be a little angry in older individuals,” Gate said. “We think this anger might make these cells less functional, resulting in dysregulation of the brain’s immune system.”
In the cognitively impaired group, inflamed T-cells cloned themselves and flowed into the CSF and brain as if they were following a radio signal, Gate said. Scientists found the cells had an overabundance of a cell receptor — CXCR6 — that acts as an antenna. This receptor receives a signal — CXCL16 — from the degenerating brain’s microglia cells to enter the brain.
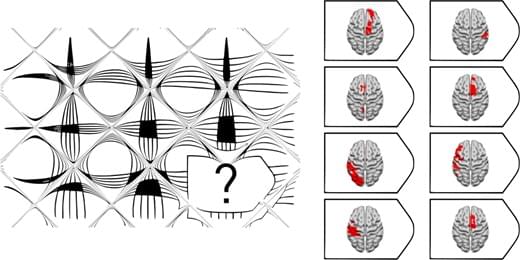
Fluid intelligence refers to the ability to solve challenging novel problems when prior learning or accumulated experience are of limited use. 1 Fluid intelligence ranks amongst the most important features of cognition, correlates with many cognitive abilities (e.g. memory), 2 and predicts educational and professional success, 3 social mobility, 4 health 5 and longevity. 6 It is thought to be a key mental capacity involved in ‘active thinking’, 7 fluid intelligence declines dramatically in various types of dementia 8 and reflects the degree of executive impairment in older patients with frontal involvement. 9 Despite the importance of fluid intelligence in defining human behaviour, it remains contentious whether this is a single or a cluster of cognitive abilities and the nature of its relationship with the brain. 10
Fluid intelligence is traditionally measured with tests of novel problem-solving with non-verbal material that minimize dependence on prior knowledge. Such tests are known to have strong fluid intelligence correlations in large-scale factor analyses. 11, 12 Raven’s Advanced Progressive Matrices 13 (APM), a test widely adopted in clinical practice and research, 14 contains multiple choice visual analogy problems of increasing difficulty. Each problem presents an incomplete matrix of geometric figures with a multiple choice of options for the missing figure. Less commonly, verbal tests of fluid intelligence such as Part 1 of the Alice Heim 4 (AH4-1) 15 are adopted. The Wechsler Adult Intelligence Scale (WAIS) 16 has also been used to estimate fluid intelligence by averaging performance on a diverse range of subtests. However, several subtests (e.g. vocabulary) emphasize knowledge, disproportionately weighting measures of ‘crystallized’ intelligence, 17, 18 whilst others (e.g. picture completion) have rather low fluid intelligence correlations. 19 Hence, it has been argued that tests such as the APM are the most suitable for a theoretically-based investigation of changes in fluid intelligence after brain injury. 20, 21
Proposals regarding the neural substrates of fluid intelligence have suggested close links with frontal and parietal functions. For example, Duncan and colleagues 22 have argued that a network of mainly frontal and parietal areas, termed the ‘multiple-demand network’ (MD), is ‘the seat’ of fluid intelligence. The highly influential parieto-frontal integration theory (P-FIT), based largely on neuroimaging studies of healthy subjects, posits that structural symbolism and abstraction emerge from sensory inputs to parietal cortex, with hypothesis generation and problem solving arising from interactions with frontal cortex. Once the best solution is identified, the anterior cingulate is engaged in response selection and inhibition of alternatives. 23, 24 Despite its name, P-FIT also posits occipital and temporal involvement, implying widely distributed substrates of fluid intelligence.

The last year in architecture will be remembered as one of firsts, from the world’s first “upcycled” skyscraper winning World Building of the Year to Burkina Faso-born Francis Kéré becoming the first African architect to win the coveted Pritzker Prize.
It was also a year in which we lost industry giants like Ricardo Bofill and Meinhard von Gerkan, while gaining long-awaited new landmarks like the Taipei Performing Arts Center and New York’s Steinway Tower.
With construction projects often taking years to complete, delays caused by Covid-19 are still being felt. But 2023 nonetheless promises to be a year of remarkable new openings, whether it’s the world’s second-tallest tower or an interfaith religious complex in Abu Dhabi.

In a recent study published in Cell, researchers used a multi-omics approach to profile the gut microbiomes and metabolomes of mothers and infants to determine the vertical and horizontal transmission of bacterial species and strains as well as individual genes and understand the dynamics of the gut microbiome assembly that shape the development of the infant before and after birth.
The vertical transmission of gut bacteria from mother to fetus during pregnancy and the horizontal transfer of microbes through breast milk plays a vital role in the physical and cognitive development of the infant long after birth. Studies have shown associations between the gut microbiota composition of breastmilk and the development of the infant’s immune system, as well as autoimmune conditions and allergies. Furthermore, allergies and autoimmune disorders have also been linked to exogenous proteins in infant formula.
Metabolites produced by gut microbiota are also associated with the infant’s cognitive development. However, the development of gut microbiomes and metabolomes in the perinatal stage and their role in infant development remains unclear.
Support human longevity research.
Want to donate with cryptocurrency? Lifespan.io is proud to announce the official launch of the Longevity Cause Fund in partnership with Sens Research Foundation and the Methuselah Foundation, facilitated by Angel Protocol.
100% of your donation will go directly to our three partner nonprofits fighting the diseases of aging. Half will be used for anti-aging work that is currently underway. The rest will be invested in perpetual endowments that will provide ongoing support for this work — forever. Aging affects us all. Help us help you stay healthier for longer. Make your donation today.
The first $10k will be matched by Angel Alliance!