Paleo’s CEO said there were “more aromatic compounds associated with grilled meat” present when the mammoth protein was added to plant-based burgers.



Biology is so freaking cool! So, I made a list (with fun facts and pictures) of just a small subset of the world’s many amazing ocean creatures. This makes me think: imagine if we knew as much about all these creatures as we know about the human body. I would love a set of nice thick copies of “Principles of Anatomy and Physiology” except that each one is devoted to a different oceanic organism.
cover image credit: Ryo Minemizu (for more, see https://www.ryo-minemizu.com/)
Anglerfish https://en.wikipedia.org/wiki/Anglerfish
Notes: males are tiny compared to females. In many species of anglerfish, mating occurs through males attaching to and then fusing with females such that their circulatory systems join together. The male provides sperm and is eventually absorbed into the female.
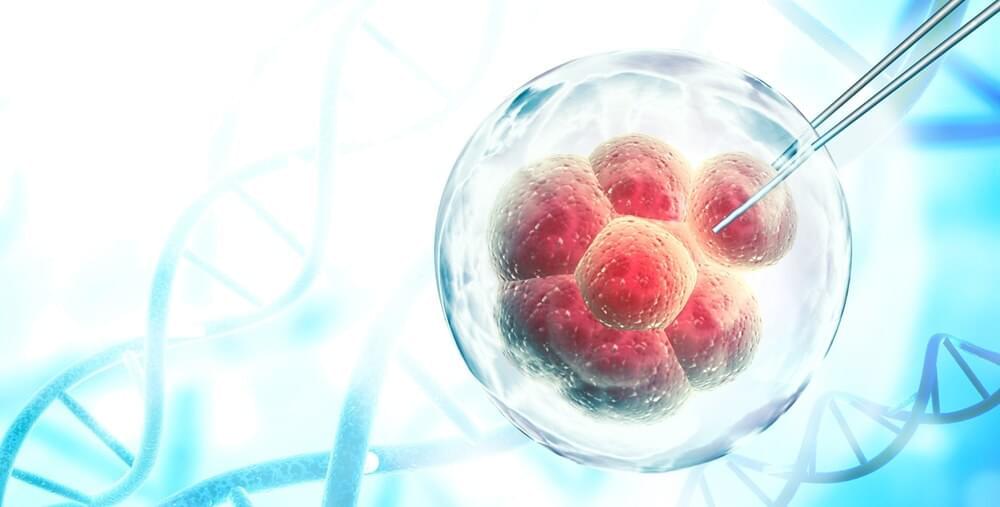
In a recent study published in the journal Cell Stem Cell, researchers hypothesized that pacemaker-like activity of human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs) resulted in engraftment arrhythmias (EAs), which hampers the clinical use of cell-based therapy using hPSC-CMs for treatment of myocardial infarction (MI).
Study: Gene editing to prevent ventricular arrhythmias associated with cardiomyocyte cell therapy. Image Credit: FrentaN / Shutterstock.
Harmful PFAS chemicals can now be detected in many soils and bodies of water. Removing them using conventional filter techniques is costly and almost infeasible. Researchers at the Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB are now successfully implementing a plasma-based technology in the AtWaPlas joint research project.
Contaminated water is fed into a combined glass and stainless steel cylinder where it is then treated with ionized gas, i.e., plasma. This reduces the PFAS molecular chains, allowing the toxic substance to be removed at a low cost.
Per-and polyfluoroalkyl substances (PFAS) have many special properties. As they are thermally and chemically stable as well as resistant to water, grease and dirt, they can be found in a large number of everyday products: Pizza boxes and baking paper are coated with them, for example, and shampoos and creams also contain PFAS. In industry they serve as extinguishing and wetting agents, and in agriculture they are used in plant protection products.
Dr. Ralph W. Moss and his son Ben discuss the anti-cancer properties and challenges of consuming green tea as a part of a healthy diet.
Program Notes:
Green tea and cancer and cardiometabolic diseases: a review of the current epidemiological evidence — https://www.nature.com/articles/s41430-020-00710-7
The Bairati Study of Antioxidants and Radiation – 2005 and 2008
The Bairati Study of Antioxidants and Radiation – 2005 and 2008
The Moss Method™ – Guide to Cancer Drug and Supplement Conflicts.
The Moss Method™ – Guide to Cancer Drug and Supplement Conflicts
Olive Oil & Cancer.

Many people who have come close to death or have been resuscitated report a similar experience: Their lives flash before their eyes, memorable moments replay, and they may undergo an out-of-body experience, sensing they’re looking at themselves from elsewhere in the room. Now, a small study mapping the brain activity of four people while they were dying shows a burst of activity in their brains after their hearts stop.
The authors say the finding, published today in the, may explain how a person’s brain could replay conscious memories even after the heart has stopped. It “suggests we are identifying a marker of lucid consciousness,” says Sam Parnia, a pulmonologist at New York University Langone Medical Center who was not involved in the study.
Although death has historically been medically defined as the moment when the heart irreversibly stops beating, recent studies have suggested brain activity in many animals and humans can continue for seconds to hours. In 2013, for instance, University of Michigan neurologist Jimo Borjigin and team found that rats’ brains showed signs of consciousness up to 30 seconds after their hearts had stopped beating. “We have this binary concept of life and death that is ancient and outdated,” Parnia says.
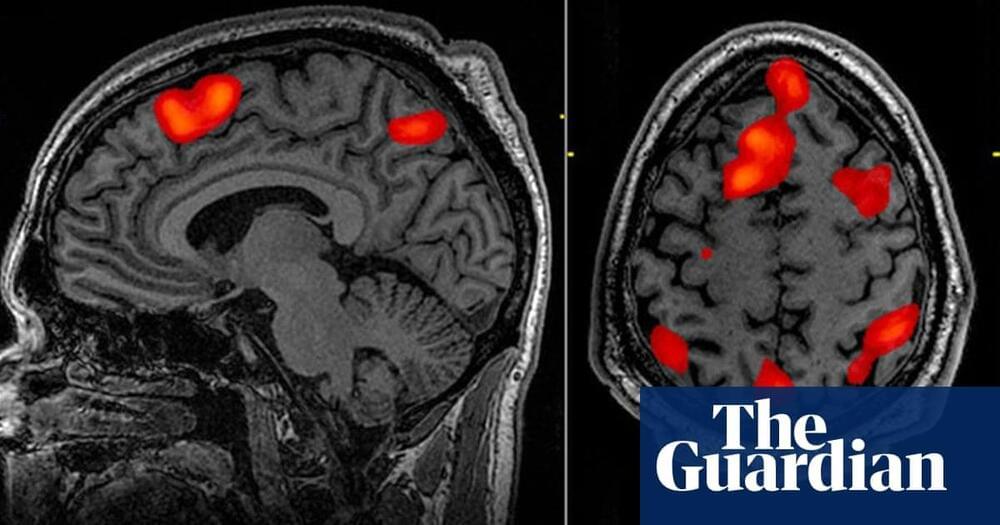
An AI-based decoder that can translate brain activity into a continuous stream of text has been developed, in a breakthrough that allows a person’s thoughts to be read non-invasively for the first time.
The decoder could reconstruct speech with uncanny accuracy while people listened to a story – or even silently imagined one – using only fMRI scan data. Previous language decoding systems have required surgical implants, and the latest advance raises the prospect of new ways to restore speech in patients struggling to communicate due to a stroke or motor neurone disease.
“For a noninvasive method, this is a real leap forward compared to what’s been done before, which is typically single words or short sentences.”
What if someone could listen to your thoughts? Sure, that’s highly improbable, you might say. Sounds very much like fiction. And we could have agreed with you, until yesterday.
Researchers at The University of Texas at Austin have decoded a person’s brain activity while they’re listening to a story or imagining telling a story into a stream of text, thanks to artificial intelligence and MRI scans.

It works for retinitis pigmentosa (RP) and dry age-related macular degeneration (AMD).
Science Corp has conceived of a new bionic eye that targets and cures two diseases that cause blindness. “Today we’re excited to take the covers off of our first flagship product development program: the Science Eye, a visual prosthesis targeted at retinitis pigmentosa (RP) and dry age-related macular degeneration (AMD), two forms of serious blindness presently without good options for patients,” said the firm in a post from November 2022.
How does it work? By targeting the functioning of the diseases.
Peshkova/iStock.
“Today we’re excited to take the covers off of our first flagship product development program: the Science Eye, a visual prosthesis targeted at retinitis pigmentosa (RP) and dry age-related macular degeneration (AMD), two forms of serious blindness presently without good options for patients,” said the firm in a post from November 2022.
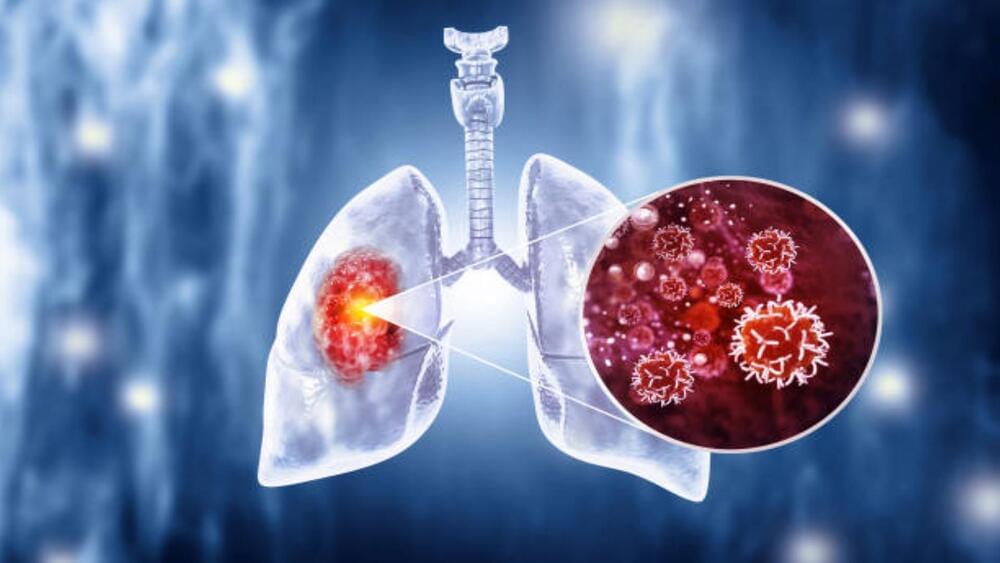
The AI model employs radiomics, a technique for extracting critical information from medical images that are not always visible to the naked eye.
An artificial intelligence (AI) model that accurately identifies cancer has been developed by a team of scientists, doctors, and researchers from Imperial College London, the Institute of Cancer Research in London, and the Royal Marsden NHS Foundation Trust.
Reportedly, this new AI model uses radiomics, a technique that extracts critical information from medical images that may not be visible to the naked eye. This, in turn, aids in determining whether the abnormal growths detected on CT scans are cancerous.