To improve upon this technology, researchers created a souped-up MRI outfitted with a high-powered 9.4-tesla magnet. (For comparison, most MRIs are equipped with a 1.5-to 3-tesla magnet.) They also added gradient coils that are 100 times stronger than current models and are what create the images, as well as a high-speed computer that is as powerful as approximately 800 laptops, according to the statement.
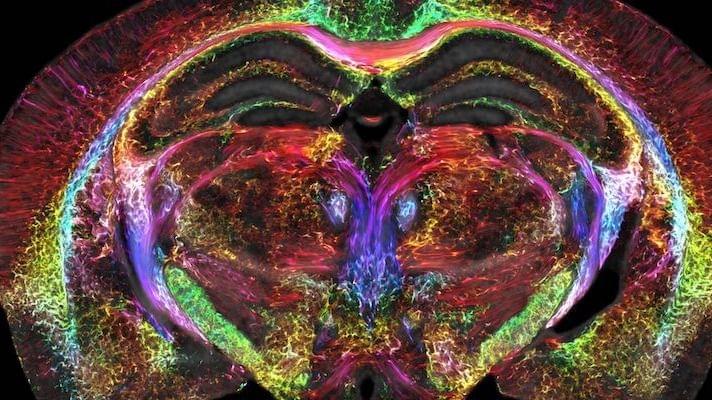
After scanning the mouse brain, the researchers sent tissue samples to be imaged using a technique called light sheet microscopy, which allowed them to label specific groups of cells in the brain that were then mapped onto the original MRI. These additional steps provided a colorful view of cells and circuits throughout the brain, according to the statement.
The researchers took one set of MRI images that captured how the mouse’s brain-wide connectivity evolved with age. A second group of images showcased brilliantly colored brain connections that highlighted the deterioration of neural networks in a rodent model of Alzheimer’s disease, according to the statement.