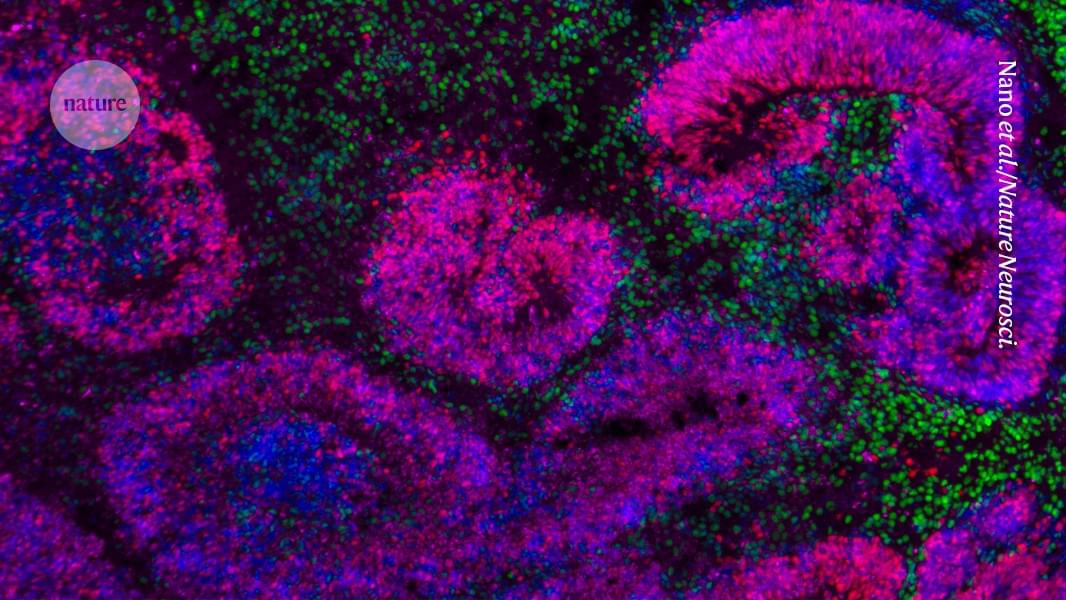
In an effort to address these ethical grey areas, 17 leading scientists and bioethicists from five countries are urging the establishment of an international oversight body to monitor advances in the rapidly expanding field of human neural organoids and to provide ethical and policy guidance as the science continues to evolve. The call to action, published Thursday in Science, comes as U.S. government agencies are making new investments in organoid science aimed at accelerating drug discovery and reducing reliance on animal models of disease.
In September, the National Institutes of Health announced $87 million in initial contracts to establish a new center dedicated to standardizing organoid research. The move followed an earlier pledge by both the NIH and the Food and Drug Administration to reduce, and possibly replace, testing on mice, primates, and other animals with other methods — including organoids and organ-on-a-chip technologies — for developing certain medicines.
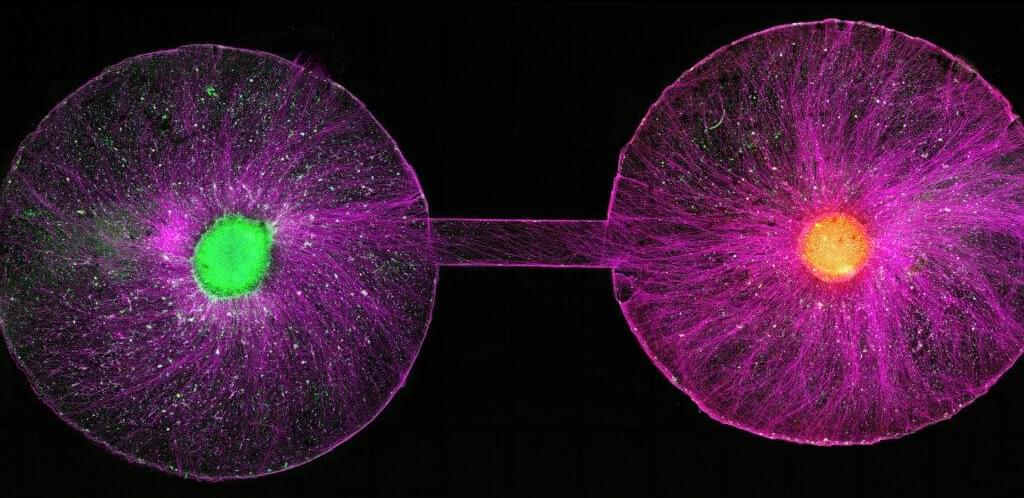
Government promotion of human stem cell models more broadly will only increase the recruitment of new researchers into the field of neural organoids, which has seen an explosion from a few dozen labs a decade ago to hundreds around the world now, said Sergiu Pasca, a pioneering neuroscientist and stem cell biologist at Stanford University who co-authored the Science commentary.