Even-aged forest management is geared towards timber production with ecosystem health as a lesser consideration. This creates a dichotomy where forests are treated either as plantations or reserves. Uneven-aged management can bring compromise to conflicting land uses by reducing ecosystem impacts while still allowing timber extraction. Whereas selection forestry focuses on which trees are taken, retention forestry focuses on protecting features that will remain after logging. These biological legacies provide ecosystem continuity.
Retained trees are often chosen based on their habitat value. Snags and living trees that are diseased, damaged, or dying provide cavities, decaying wood, and other microhabitats for a diversity of biota. Defects that make high-quality habitat trees tend to cause the collapse of large and old trees, so it’s important to designate healthy recruitment trees for the future. Retention forestry that focuses only on habitat trees may be inconsistent with the goals of long-term carbon storage and ecosystem resilience.
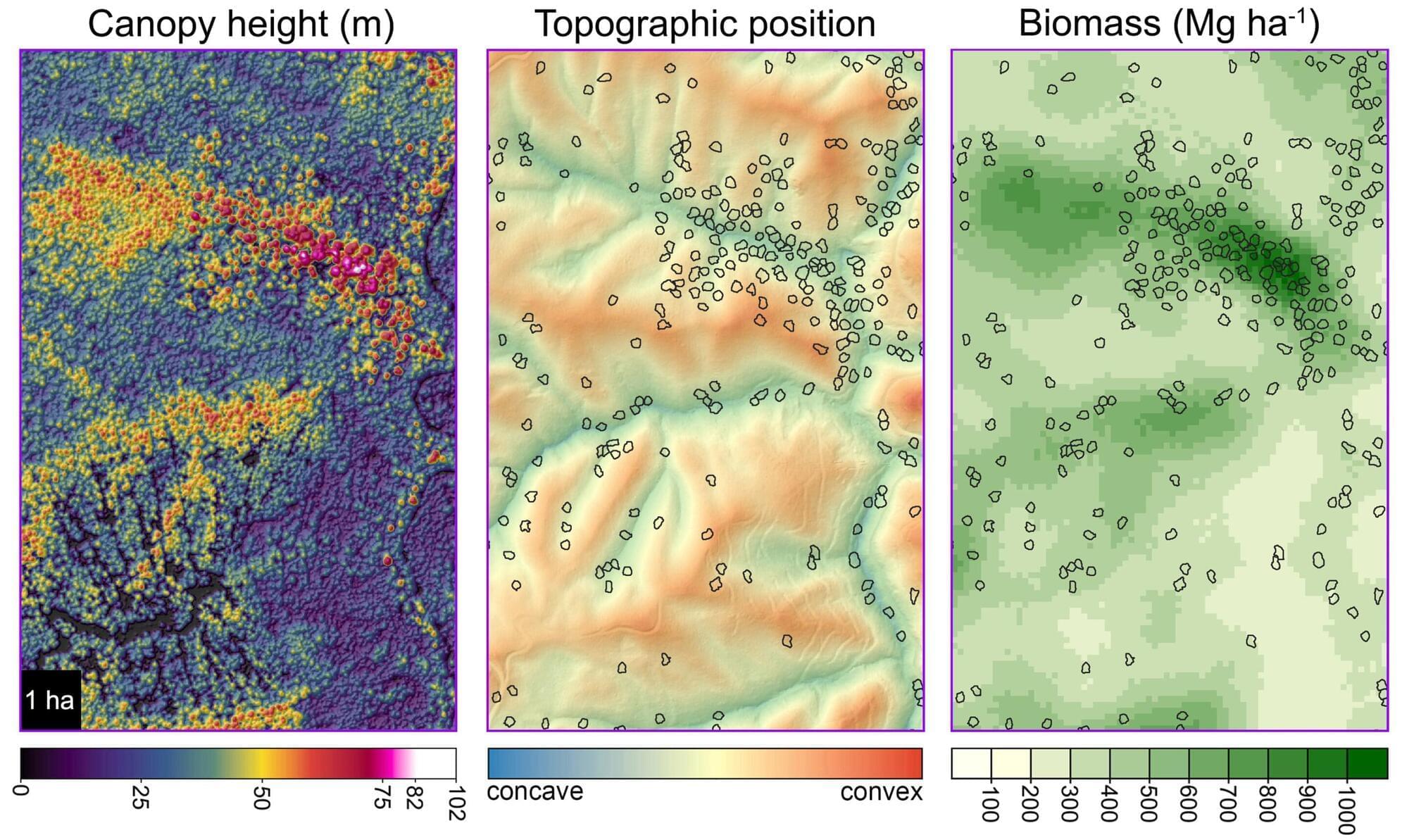
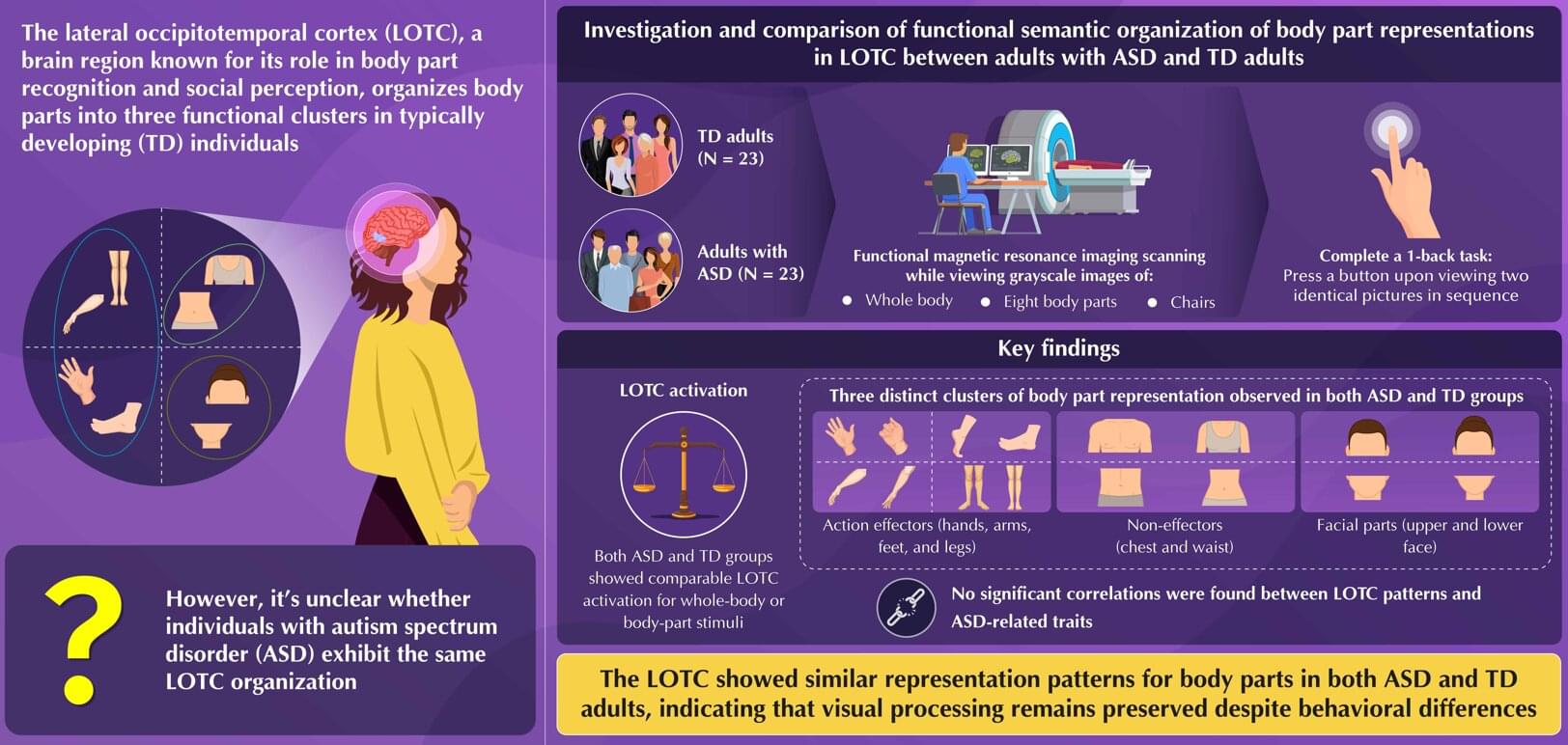
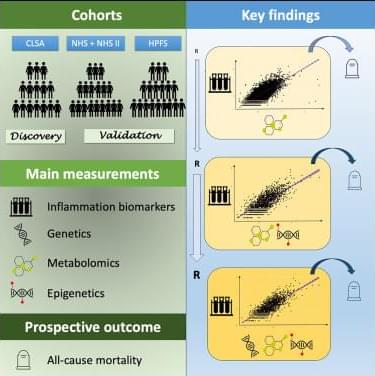
An article just published in Forest Ecology and Management explores the idea of “exceptional trees” and why we might consider choosing a subset of the most robust trees for permanent retention in managed forests. We present methods for precisely estimating aboveground biomass across the landscape and assess the contribution of exceptional trees to biomass and productivity. Our study focuses on Sequoia sempervirens (redwood) in California’s Demonstration State Forests.