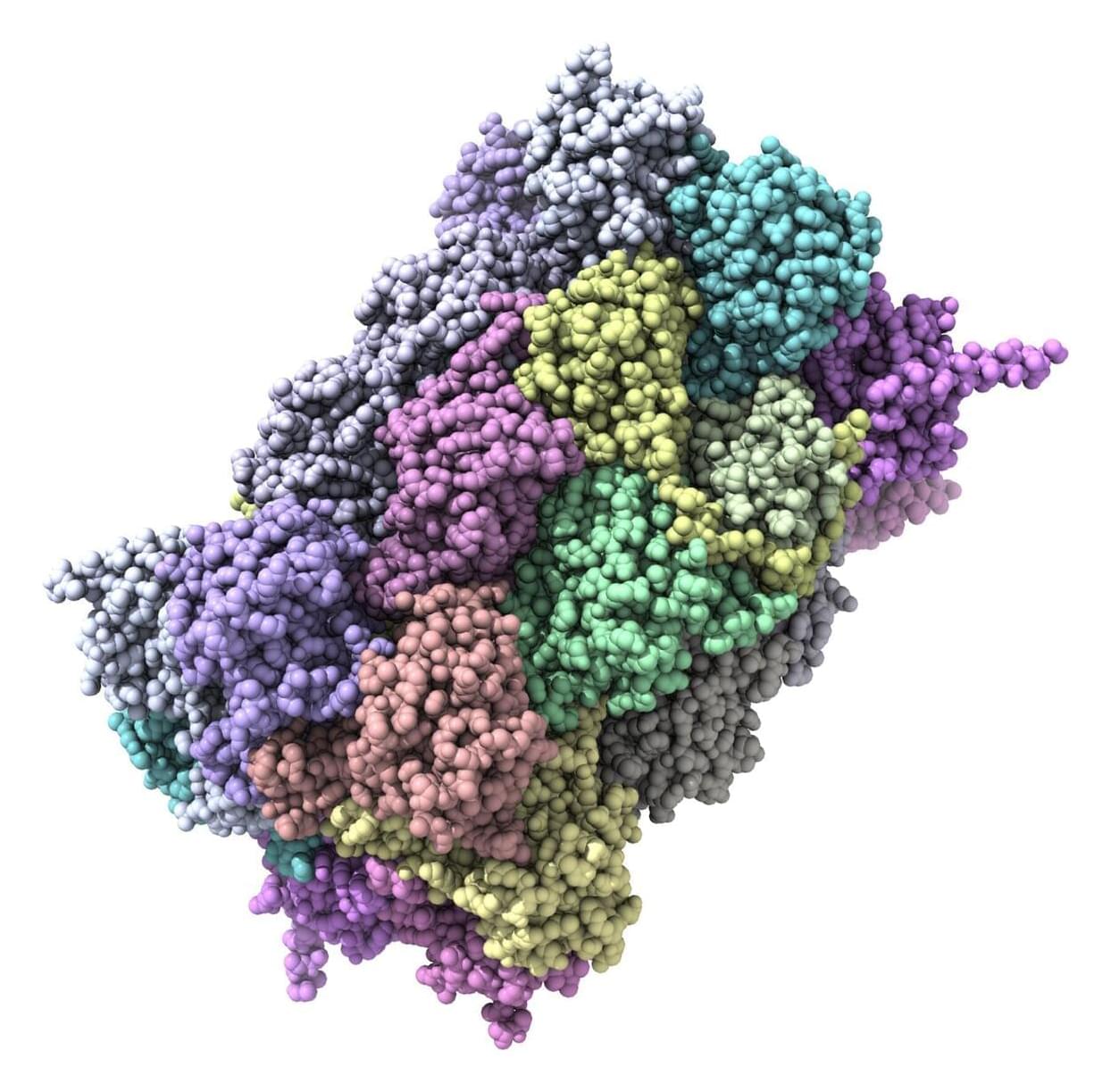
A new microchip invented by Scripps Research scientists can reveal how a person’s antibodies interact with viruses—using just a drop of blood. The technology offers researchers faster, clearer insights that could help accelerate vaccine development and antibody discovery.
“This lets us take a quick snapshot of antibodies as they are evolving after a vaccine or pathogen exposure,” says Andrew Ward, professor in the Department of Integrative Structural and Computational Biology at Scripps Research and senior author of the new paper published in Nature Biomedical Engineering on June 3, 2025. “We’ve never been able to do that on this timescale or with such tiny amounts of blood before.”
When someone is infected with a virus, or receives a vaccine, their immune system creates new antibodies to recognize the foreign invader. Some antibodies work well against the pathogen, while others attach to it only weakly. Figuring out exactly which parts of the virus the best antibodies stick to is key information for scientists trying to optimize vaccines, since they want to design vaccines that elicit strong, reliable immune responses.