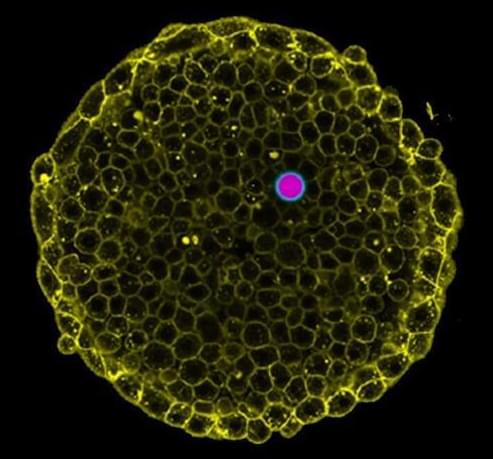
In order to survive, organisms must control the pressure inside them, from the single-cell level to tissues and organs. Measuring these pressures in living cells and tissues in physiological conditions is a challenge.
In research that has its origin at UC Santa Barbara, scientists now at the Cluster of Excellence Physics of Life (PoL) at the Technical University in Dresden (TU Dresden), Germany, report in the journal Nature Communications a new technique to ‘visualize’ these pressures as organisms develop. These measurements can help understand how cells and tissues survive under pressure, and reveal how problems in regulating pressures lead to disease.
When molecules dissolved in water are separated into different compartments, water has the tendency to flow from one compartment to another to equilibrate their concentrations, a process known as osmosis. If some molecules cannot cross the membrane that separates them, a pressure imbalance—osmotic pressure—builds up between compartments.