Adversity experienced by mothers during their childhood or pregnancy is reflected in their children’s gut microbiomes, a recent study found.


Our gut microorganisms, collectively known as the gut microbiota, affect far more than digestion. Bacteria in our intestinal tracts influence brain activity — and even the likelihood of developing mental disorders. Decades of research have shown that a bacterially imbalanced gut can disrupt many systems in the human body, contributing to obesity, malnutrition and even cancer. In a study published May 10 in Nature, Stanford Medicine researchers and collaborators used an ingestible device to capture the diversity of microorganisms, viruses, proteins and bile in the small intestine.
The proof-of-concept results provide early evidence that there are more comprehensive ways to measure microbiota in the digestive system than current sampling methods — which mostly focus on stool — and shed new light on how resident gut microbes might contribute to human physiology and disease.
“This paper demonstrates a big leap forward in microbial detection and captures the living gut microbiota in a nutshell,” said co-senior author KC Huang, PhD, a professor of bioengineering and of microbiology and immunology, co-senior author with David Relman, MD, a professor of medicine and of microbiology and immunology. “Samples from current tools don’t fully represent what’s going on inside of us. But it’s all we’ve had — until now.”
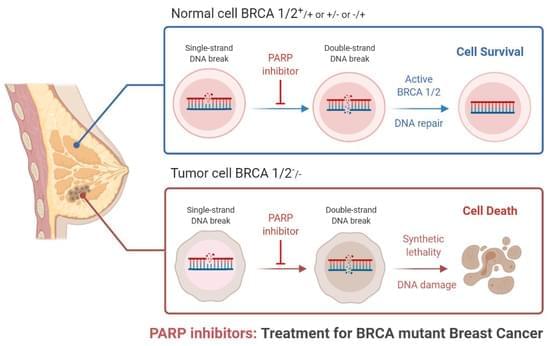
Triple-negative breast cancer is a combative cancer type with a highly inflated histological grade that leads to poor theragnostic value. Gene, protein, and receptor-specific targets have shown effective clinical outcomes in patients with TNBC. Cells are frequently exposed to DNA-damaging agents. DNA damage is repaired by multiple pathways; accumulations of mutations occur due to damage to one or more pathways and lead to alterations in normal cellular mechanisms, which lead to development of tumors. Advances in target-specific cancer therapies have shown significant momentum; most treatment options cause off-target toxicity and side effects on healthy tissues.
Novel Harm Reduction Strategies For The Opioid Epidemic — Harmguard FX — Jermonica Boardley & Joseph Scrocco — Sivad Group LLC / Diagnostic Solutions Group
Jermonica Boardley is President and CEO of SIVAD Diagnostic Medical Group LLC (https://sivadppe.com/), a minority-owned company dedicated to addressing the unique needs of underserved and unserved communities in the United States and globally, which most recently announced the launch of HarmGuard FX (https://www.harmstopper.com/), a single, low-cost strip that provides an affordable, reliable way for people to test substances for fentanyl and xylazine (popularly known as tranq), two dangerous adulterants that are exacerbating the already critical opioid epidemic.
Ms. Boardley is a graduate of Delaware State University, brings over 20 years of diverse corporate leadership experience to her role as President and CEO and her expertise spans Accounting, Finance, Human Resources, Communications, and Diversity, Equity & Inclusion (DE&I).
Joseph Scrocco, is President & CEO, Diagnostic Solutions Group (https://www.diagnosticsolutionsgroup.com/), the developer of HarmGuardFX, and their company has developed custom diagnostic solutions for over 25 years ranging from complex mass screening for blood banks to individual point-of-care testing with a focus on large-scale, global diagnostic needs, including antibody, molecular, and antigen tests in traditional and non-traditional settings. Their clients include Johnson & Johnson; Pfizer; Novo Nordisk; Abbott Labs; Ortho Clinical Diagnostics; the Red Cross; the governments of Nigeria, Ivory Coast, Bangladesh, Liberia, Jamaica, India, Mali; numerous Broadway companies; plus, hundreds of hospitals, urgent care centers, and long-term care facilities.
Mr. Scrocco has a MS in Neurophysiology from University of Medicine and Dentistry of New Jersey, and an MA in Information Systems from New York University.
Alex’s lemonade stand foundation — fighting childhood cancer, one cup at a time! liz scott, co-executive director, alex’s lemonade stand foundation.
Liz Scott is Co-Executive Director, of Alex’s Lemonade Stand Foundation for Childhood Cancer (ALSF — https://www.alexslemonade.org/), an organization which emerged from the front yard lemonade stand of her daughter, Alexandra “Alex” Scott (1996−2004).
In 2000, at the age of four, Alex announced that she wanted to hold a lemonade stand to raise money to help find a cure for other children with cancer, herself already bravely fighting neuroblastoma, which she was diagnosed with at age one.
Over her lifetime, Alex would raise over $1 million before she passed away in 2004 at the age of 8. Since then, Liz and her husband Jay have worked alongside thousands of supporters across the country to carry on Alex’s legacy of hope.
To date, ALSF has raised more than $250 Million toward fulfilling Alex’s dream of finding a cure, funding over 1,000 pediatric cancer research projects nationally.

In March 2020, an experiment in science philanthropy was hatched in the span of a five-minute call.
Patrick Collison, the now 34-year-old billionaire CEO of the online payments company Stripe, and economist Tyler Cowen were chewing over a shared concern: Scientific progress seemed to be slowing down. As the first pandemic lockdowns went into effect, researchers were in a holding pattern, waiting to hear if they could redirect their federal grants to COVID-related work. Collison and Cowen worried that the National Institutes of Health wasn’t moving quickly enough, so they launched Fast Grants to get emergency research dollars to virologists, coronavirus experts, and other scientists rapidly.
“We thought: Let’s just do this,” Cowen recalls. “It was a bit like put up or shut up.”


If you’re fighting cancer, a healthy lifestyle can help you stay well during and after treatment. Research suggests that good nutrition, exercise and other healthy behaviors may improve your quality of life during treatment.
“Taking charge of your health by developing healthy habits empowers you to cope better with your treatment and any side effects you may have,” says Lonny Yarmus, D.O., a board certified interventional pulmonologist at the Lung Cancer Program at the Sidney Kimmel Comprehensive Cancer Center.

In a recent perspective piece published in the Nature Medicine Journal, researchers discussed the current achievements, challenges, and potential opportunities in using digital technologies, such as remote medicine and wearables in geriatric medicine and care.
Study: Digital health for aging populations. Image Credit: GroundPicture/Shutterstock.com.
A natural sugar called mannose is a type of hexose that is abundant in many different types of fruits. Recent studies have demonstrated that mannose has been found to be effective in promoting immune tolerance, suppressing inflammatory diseases, and efficient in suppressing tumors by suppressing glycolysis. However, it is not fully understood how mannose exerts its anticancer activity. Now, a study by Sanford Burnham Prebys and the Osaka International Cancer Institute has shed new light on the anticancer properties of mannose and suggests that mannose could be a helpful secondary treatment for cancer.
The findings are published in eLife in an article titled, “Metabolic clogging of mannose triggers dNTP loss and genomic instability in human cancer cells.”
“Mannose has anticancer activity that inhibits cell proliferation and enhances the efficacy of chemotherapy,” wrote the researchers. “How mannose exerts its anticancer activity, however, remains poorly understood. Here, using genetically engineered human cancer cells that permit the precise control of mannose metabolic flux, we demonstrate that the large influx of mannose exceeding its metabolic capacity induced metabolic remodeling, leading to the generation of slow-cycling cells with limited deoxyribonucleoside triphosphates (dNTPs).”