The first successful transplantation of a cryopreserved rat kidney has indicated that long-term storage of human organs for transplantation may be possible.


“The other thing we might find is actually this long flow, [this] drip of pharmaceuticals: caffeine, lidocaine, cocaine, amphetamine, antidepressants, birth control — this long slow drift of them from cities into the [ocean] is… starting to hit these animals,” Hird said.
Shark Week show delves into whether sharks off the coast of Florida are coming into contact with the huge quantities of cocaine that get dumped in these waters.

Integrated Biosciences, a biotechnology company combining synthetic biology and machine learning to target aging, in collaboration with researchers at the University of California Santa Barbara, today announced a drug discovery platform that enables precise control of the integrated stress response (ISR), a biological pathway that is activated by cells in response to a wide variety of pathological and aging-associated conditions.
A new publication, “Optogenetic control of the integrated stress response reveals proportional encoding and the stress memory landscape,” authored by company founders and featured on the cover of Cell Systems describes a technique that triggers the ISR virtually using light and demonstrates how the accumulation of stress over time shifts a cell’s reaction from adaptation to apoptosis (programmed cell death).
“In a very real way, our platform puts cells into a virtual reality, making them experience stress in the absence of physical stressors,” said Maxwell Wilson, Ph.D., a co-founder of Integrated Biosciences and Assistant Professor of Molecular, Cellular, and Developmental Biology at the University of California Santa Barbara.
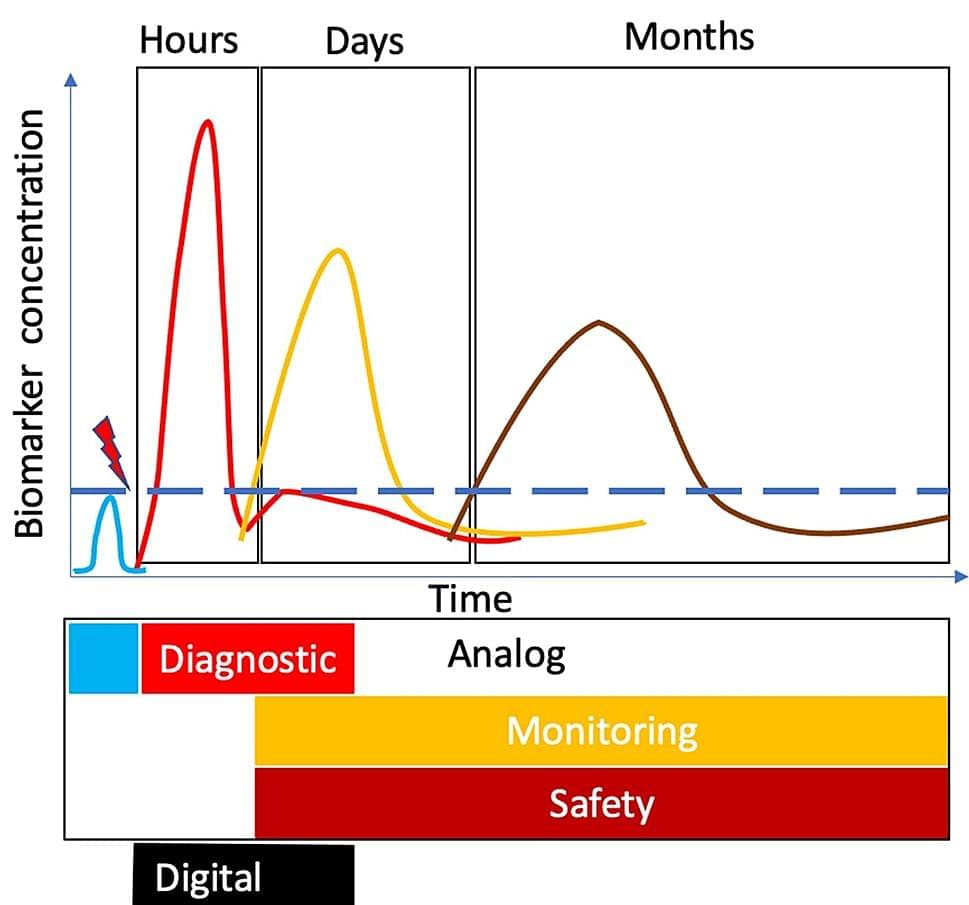
Traumatic brain injury (TBI) is increasingly a major cause of disability across the globe. The current methods of diagnosis are inadequate at classifying patients and prognosis. TBI is a diagnostic and therapeutic challenge. There is no Food and Drug Administration (FDA)-approved treatment for TBI yet. It took about 16 years of preclinical research to develop accurate and objective diagnostic measures for TBI. Two brain-specific protein biomarkers, namely, ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein, have been extensively characterized. Recently, the two biomarkers were approved by the FDA as the first blood-based biomarker, Brain Trauma Indicator™ (BTI™), via the Breakthrough Devices Program. This scoping review presents (i) TBI diagnosis challenges, (ii) the process behind the FDA approval of biomarkers, and (iii) known unknowns in TBI biomarker biology.
We know a lot about cancer, and yet, there is plenty we do not yet know. We do know that some cancers are genetic in nature and a series of changes in key genes can lead to identifiable malignancies down the line. We would certainly want to know what causes cancer in the first place.
Scientists have been trying to replicate the path a cell takes from being normal to becoming pre-cancerous (one of the earliest stages of cancer in which cells become abnormally shaped and sized) for quite some time now. It is a feat that requires human-derived cells to model how cancer comes to be.
Recently, researchers at The Stanford School of Medicine have been able to emulate some of the earliest stages of gastric cancer by starting with gastric organoids (a rudimentary version of the real stomach made from stem-cell-derived gastric cells) that have a single mutation. The study which was published in Nature outlines how the earliest changes in cells could be seen even before the precancerous stage.

There’s an exciting new development in the field of Alzheimer’s disease research. Surprisingly, it centers around a common bodybuilding supplement known as HMB. The key to preserving memory and staving off this devastating disease may, in fact, reside in the diet of those pumping iron at the gym.
Researchers from RUSH Medical College have recently revealed that the muscle-enhancing supplement known as beta-hydroxy beta-methylbutyrate (HMB) could hold potential in the fight against Alzheimer’s disease.
The supplement, frequently used by bodybuilders to boost muscle growth and enhance performance, might also aid in memory protection, plaque reduction, and slowing the progression of Alzheimer’s disease.
New research provides evidence that an individual’s health behaviors and outcomes are influenced by the genetic makeup of their romantic partner. The findings, published in Behavior Genetics, indicate that your partner’s genetic tendencies can lead to changes in your own weight, smoking habits, or alcohol consumption over time.
The researchers conducted this study to investigate how a person’s partner can affect their health. They aimed to explore the concept of social genetic effects, which refers to the impact of genetic factors in one person’s environment, such as their partner’s genotype, on their own phenotype (observable characteristics or traits).
“I was mainly interested in exploring the combination of social science and genetics,” explained study author Kasper Otten of Utrecht University. “It is evident that behavior is partly genetically influenced, but much of the social sciences does not deal with this biological fact.

Milk and colostrum have high biological potential, and due to their natural origin and non-toxicity, they have many uses in cosmetics and dermatology. Research is ongoing on their potential application in other fields of medicine, but there are still few results; most of the published ones are included in this review. These natural products are especially rich in proteins, such as casein, β-lactoglobulin, α-lactalbumin, lactoferrin, immunoglobulins, lactoperoxidase, lysozyme, and growth factors, and possess various antibacterial, antifungal, antiviral, anticancer, antioxidant, immunomodulatory properties, etc. This review describes the physico-chemical properties of milk and colostrum proteins and the natural functions they perform in the body and compares their composition between animal species (cows, goats, and sheep). The milk-and colostrum-based products can be used in dietary supplementation and for performing immunomodulatory functions; they can enhance the effects of certain drugs and can have a lethal effect on pathogenic microorganisms. Milk products are widely used in the treatment of dermatological diseases for promoting the healing of chronic wounds, hastening tissue regeneration, and the treatment of acne vulgaris or plaque psoriasis. They are also increasingly regarded as active ingredients that can improve the condition of the skin by reducing the number of acne lesions and blackheads, regulating sebum secretion, ameliorating inflammatory changes as well as bestowing a range of moisturizing, protective, toning, smoothing, anti-irritation, whitening, soothing, and antiaging effects.
Keywords: milk, colostrum, casein, β-lactoglobulin, α-lactalbumin, lactoferrin, growth factors, skin, regeneration, antimicrobial, cosmetics.
Although milk is known to be used as a raw material in the food industry, it is also widely used in the pharmaceutical and cosmetic industries due to its considerable biological potential. It has also been the subject of detailed analyses and discussions of its individual components and their properties [1, 2].
A CAR T-cell therapy targeting disease-driving immune cells safely led to sustained disease remission for five people with systemic lupus erythematosus (SLE) who’d previously failed to respond to other treatments, a recent study reported.
Treatment was also highly specific, preventing autoimmune activity, but didn’t impair general immune system function.
“These data provide new therapeutic possibilities to control SLE disease activity,” the researchers wrote. “Longer follow-ups in larger cohorts of patients will be necessary to confirm sustained absence of autoimmunity and resolution of inflammation in patients with SLE who have received CAR T cell therapy.”
Nature Abstract.
https://www.nature.com/articles/s41591-022-02017-5
No systemic lupus erythematosus patient receiving the CAR T-cell therapy infusion relapsed, all remained in remission for up to 17 months.