A team of scientists used gene editing to create what they thought would be a calmer rodent. Instead, the gene-edited rodents were angrier.



A new Seattle biotech organization will be funded to the tune of $75 million to research “DNA typewriters,” self-monitoring cells that could upend our understanding of biology. The collaboration between the University of Washington, the Chan-Zuckerberg Initiative and the Allen Institute is already underway.
Called the Seattle Hub for Synthetic Biology, the joint initiative will combine the expertise of the two well-funded research outfits with that of UW Medicine, working in what UW’s Jay Shendure, scientific lead for the project, called “a new model of collaboration.”
The Hub (not to be confused with the HUB, or Husky Union Building, on UW’s campus) aims to strike a balance between a disinterested intellectual academic approach and a development-focused commercial approach. The $75 million will fund the organization for five years, with the option to renew then.
Good telescope that I’ve used to learn the basics: https://amzn.to/35r1jAk.
Get a Wonderful Person shirt: https://teespring.com/stores/whatdamath.
Alternatively, PayPal donations can be sent here: http://paypal.me/whatdamath.
Hello and welcome! My name is Anton I’m away for a few days due to voice issues, so enjoy this older video where we talk about the incredible invention of 3D printed bio ink that could be used to print any biological tissue (in theory). 3D printed heart anyone?
Links:
https://www.nature.com/articles/s41467-021-26791-x.
https://www.mdpi.com/2072-666X/12/8/865
https://www.sciencedaily.com/releases/2021/09/210921134345.htm.
https://en.wikipedia.org/wiki/Fibrin.
Bladder grown from 3D bioprinted tissue continues to function after 14 years
https://www.ascb.org/science-news/bioprinting-ethical-and-societal-implications/
Biocomputing: https://youtu.be/nszcPNhYRzI
Artificial cell: https://youtu.be/0MRGJNKACYs.
Synthethic genome: https://youtu.be/OxVZPKmm58M
0:00 History of 3D printing organs.
2:00 Why this is important for medical studies.
2:45 Bioink invention.
3:40 How this works.
5:30 Results from the study are quite incredible.
6:30 Future of medical 3D printing.
Support this channel on Patreon to help me make this a full time job:
https://www.patreon.com/whatdamath.
Bitcoin/Ethereum to spare? Donate them here to help this channel grow!
bc1qnkl3nk0zt7w0xzrgur9pnkcduj7a3xxllcn7d4
or ETH: 0x60f088B10b03115405d313f964BeA93eF0Bd3DbF
Space Engine is available for free here: http://spaceengine.org.



Target validation is a crucial step in pre-clinical drug discovery workflows that builds confidence on the identification of a genetic target as relevant to a disease. With recent advancements, CRISPR serves as a particularly powerful tool for this process, as it enables researchers to accurately modify genes and determine their function in a variety of experimental systems.
One scientist leveraging CRISPR gene editing in this way is Dr. Panos Zalmas, Head of the Open Targets Validation Lab based at the Wellcome Sanger Institute, whose work focuses on discovering and validating new putative disease targets for the development of safe and effective medicines.
In this SelectScience® interview, we speak with Zalmas to learn how he is working to improve the rate of target adoption into drug discovery pipelines across therapy areas such as oncology, neurodegeneration, and immunology and inflammation. Here, Zalmas explains the importance of gene editing in his target validation workflows and highlights how CRISPR technologies in particular are key to the success of drug discovery.
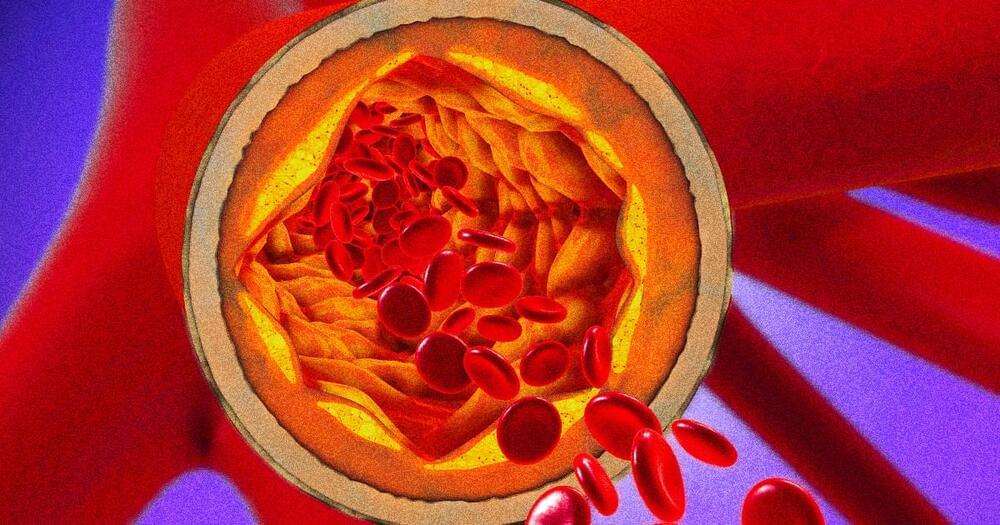
Researchers have been able to reduce dramatically the level of bad cholesterol in human subjects after injecting them with an experimental gene editing treatment, according to the science journal Nature, which is the first time this technique, called base editing, has been done on humans.
But at least one person died after receiving an infusion, prompting a round of safety concerns.
In the clinical trial, 10 subjects with congenitally high levels of bad cholesterol, aka low-density lipoprotein (LDL), were given an injection of VERVE-101, a gene-editing treatment that uses the base editing technique. This treatment then turned off the gene for the protein PCSK9, which is found in the liver and regulates LDL. High levels of LDL can lead to coronary heart disease.

The groundbreaking gene-editing technology known as Crispr, which acts like a molecular pair of scissors that can be used to cut and modify a DNA sequence, has moved rather quickly from the pages of scientific journals to the medical setting. Earlier this month, about three years after Jennifer Doudna and Emmanuelle Charpentier won the Nobel Prize in Chemistry for describing how bacteria’s immune system could be used as a tool to edit genes, regulators in the U.K. approved the first Crispr-based treatment for sickle cell disease and beta-thalassemia patients. The treatment, from Vertex Pharmaceuticals and Crispr Therapeutics, could be approved by the U.S. Food and Drug Administration early next month for sickle cell patients.
While many obstacles lie ahead for the nascent field, such as how to pay for treatments that typically cost more than $1 million, these regulatory approvals are just the start as newer gene-editing technologies such as base and prime editing make their way through human studies. In an interview, Prof. Doudna says the approval is “a turning point in medicine because it really shows how genome editing can be used as a one-and-done cure for disease.”
Gene editing is part of a broader therapeutic revolution that encompasses genetic and cellular medicine. The pills and injections we are all familiar with generally target proteins or pathways in the body to treat disease. With gene and cell therapy, we can now target the root cause of disease, sometimes curing patients.