Recently approved gene therapies offer patients one-time, potentially curative treatments for genetic diseases such as sickle cell anemia and beta thalassemia. But “one-time” miracle solutions can often be multi-month affairs, require millions of dollars, and cause painful side effects. What if that doesn’t have to be the case?
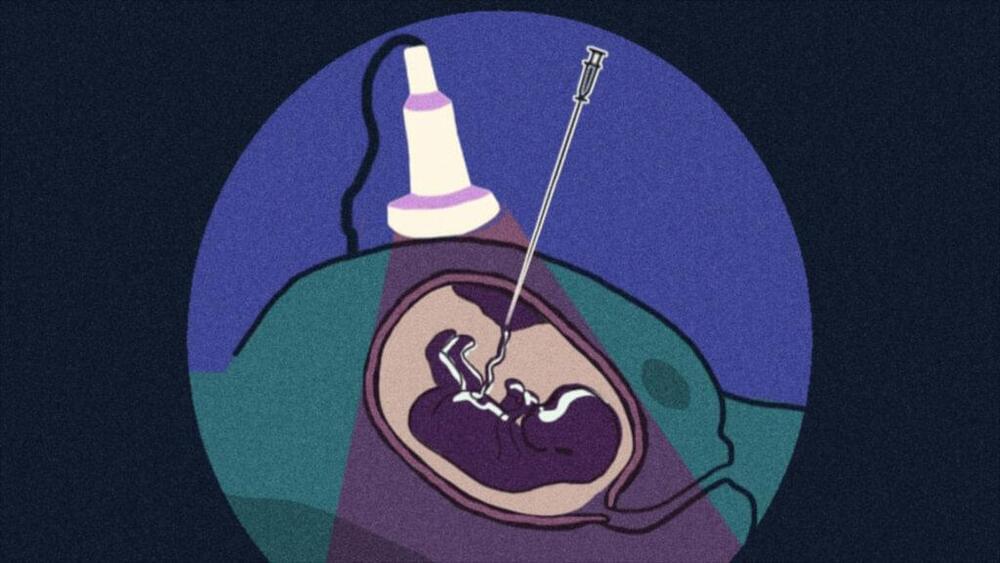
In utero gene editing, or prenatal somatic cell genome editing, envisions treating a fetus diagnosed with a genetic disease before birth, thereby preventing that entire protocol and the onset of symptoms in the first place. It would also challenge the need for the ethically fraught enterprise of embryo editing, as the treatment would only make edits in the DNA of the individual fetus — edits which would not be passed on in a heritable way.
Watch this video to learn more about in utero gene editing, how it works, and why scientists believe it might be an advantageous approach to treating certain genetic diseases.