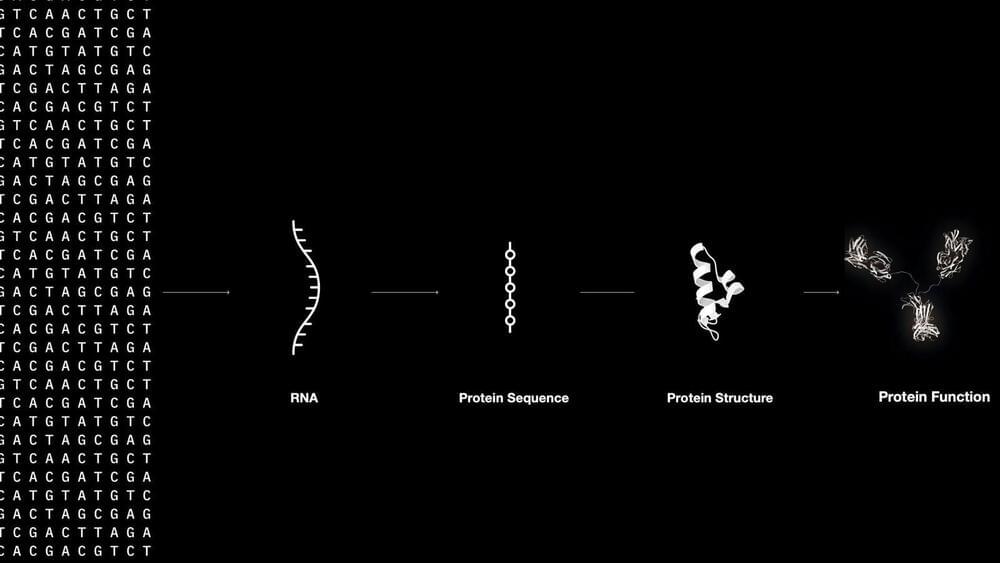
Experts can see how AI can be applied to biology to build biological systems.

A dietary supplement may offer a novel way to enhance the effectiveness of CAR T cell therapy, according to a study conducted by researchers at the Perelman School of Medicine and the Abramson Cancer Center at the University of Pennsylvania. Although this method requires validation through clinical trials, early findings—recently presented during a press briefing at the 66th American Society of Hematology (ASH) Annual Meeting and Exposition—suggest a potentially affordable and accessible strategy to improve CAR T cell functionality and cancer-fighting capabilities.
CAR T cell therapy, first developed at Penn Medicine, is a personalized cancer treatment that reprograms a patient’s immune cells to target and destroy cancer cells.
“Thousands of patients with blood cancers have been successfully treated with CAR T cell therapy, but it still doesn’t work for everyone,” said co-lead author Shan Liu, PhD, a postdoctoral fellow who presented the study at ASH. “We took an outside-the-box approach to improve CAR T cell therapy, by targeting T cells through diet rather than further genetic engineering.”
Middlemen get a bad rap for adding cost and complications to an operation. So, eliminating the go-betweens can reduce expense and simplify a process, increasing efficiency and consumer happiness.
James Dahlman and his research team have been thinking along those same lines for stem cell treatments. They’ve created a technique that eliminates noisome middlemen and could lead to new, less-invasive treatments for blood disorders and genetic diseases. It sidesteps the discomfort and risks of current treatments, making life easier for patients.
“This would be an alternative to invasive hematopoietic stem cell therapies—we could just give you an IV drip,” said Dahlman, McCamish Early Career Professor in the Wallace H. Coulter Department of Biomedical Engineering. “It simplifies the process and reduces the risks to patients. That’s why this work is important.”
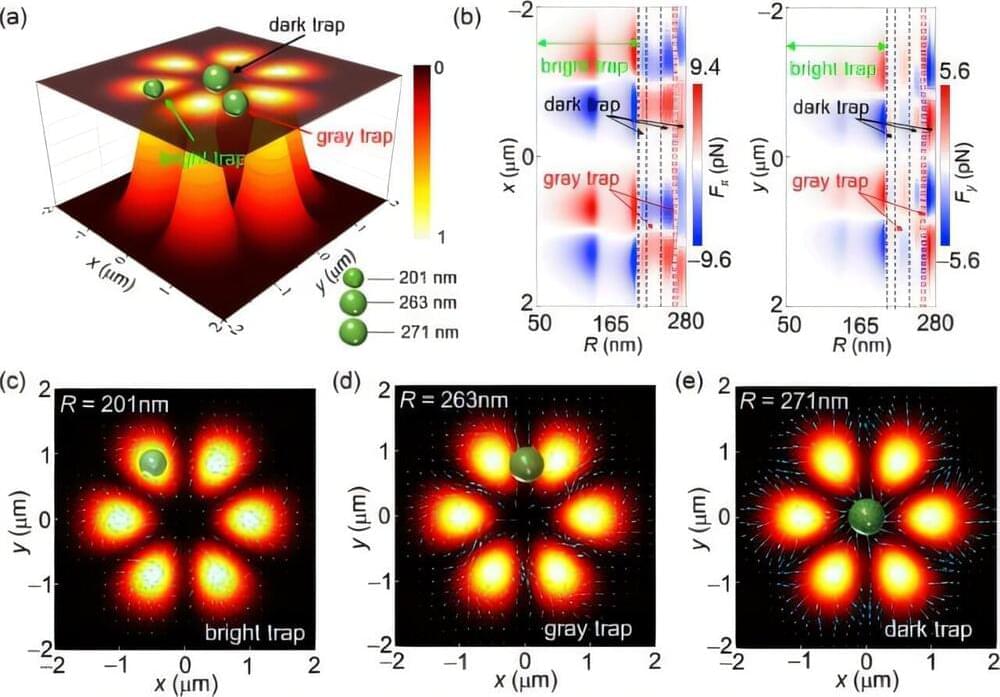
A research group led by Prof. Yao Baoli and Dr. Xu Xiaohao from Xi’an Institute of Optics and Precision Mechanics (XIOPM) of the Chinese Academy of Sciences have revealed a full-gray optical trap in structured light, which is able to capture nanoparticles but appears at the region where the intensity is neither maximized nor minimized. The study is published in Physical Review A.
The optical trap is one of the greatest findings in optics and photonics. Since the pioneering work by Arthur Ashkin in the 1970s, the optical trap has been employed in a broad range of applications in life sciences, physics, and engineering. Akin to its thermal and acoustic counterparts, this trap is typically either bright or dark, located at the field intensity maxima or minima.
In this study, researchers developed a high-order multipole model for gradient forces based on multipole expansion theory. Through immersing the Si particles in the structured light with a petal-shaped field, they found that the high-order multipole gradient forces can trap Si particles at the optical intensity, which is neither maximized nor minimized.
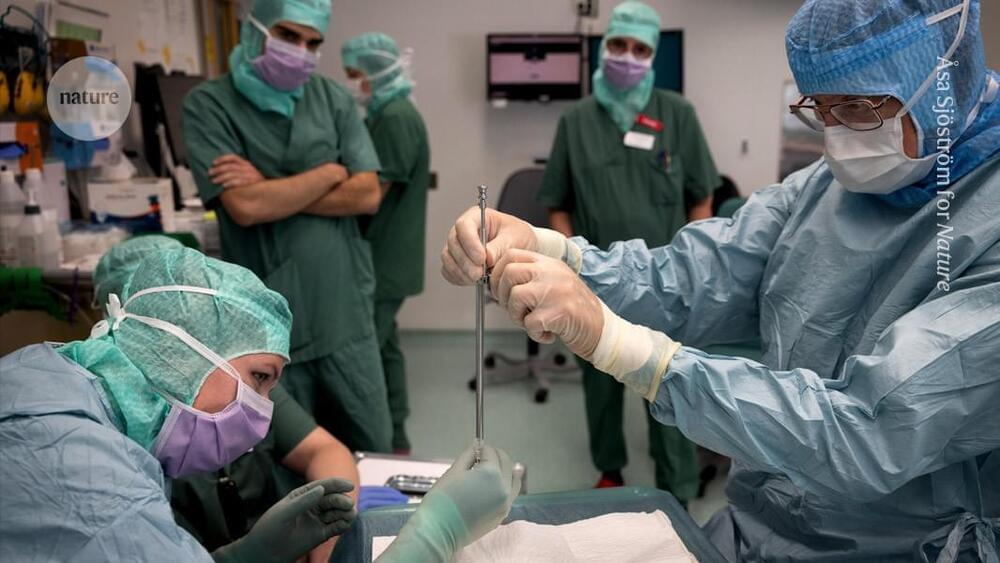
Andrew Cassy had spent his working life in a telecommunications research department until a diagnosis of Parkinson’s disease in 2010 pushed him into early retirement. Curious about his illness, which he came to think of as an engineering problem, he decided to volunteer for clinical trials.
“I had time, something of value that I could give to the process of understanding the disease and finding good treatments,” he says.
In 2024, he was accepted into a radical trial. That October, surgeons in Lund, Sweden, placed neurons that were derived from human embryonic stem (ES) cells into his brain. The hope is that they will eventually replace some of his damaged tissue.
The study is one of more than 100 clinical trials exploring the potential of stem cells to replace or supplement tissues in debilitating or life-threatening diseases, including cancer, diabetes, epilepsy, heart failure and some eye diseases. It’s a different approach from the unapproved therapies peddled by many shady clinics, which use types of stem cell that do not turn into new tissue.
More than 100 clinical trials put stem cells for regenerative medicine to the test. It’s a turning point for a field beset with ethical and political controversy.
Sometimes pain is a necessary warning signal; for example, if we touch something very hot and it burns, we know to move our hand away. But chronic pain can destroy a person’s quality of life, and it can be extremely challenging to get relief. Some researchers have been searching for ways to deactivate pain receptors, so the body no longer feels the neural signals of chronic pain. Using mouse models of acute inflammatory pain, scientists have shown that it is possible to deactivate pain receptors with genetic engineering tools. The work has been reported in Cell.
“What we have developed is potentially a gene therapy approach for chronic pain,” said senior study author Bryan L. Roth, MD, PhD, a distinguished professor at the University of North Carolina (UNC) School of Medicine, among other appointments. “The idea is that we could deliver this chemogenetic tool through a virus to the neurons that sense the pain. Then, you could just take an inert pill and turn those neurons off, and the pain will literally disappear.”
Bioconvergence — Bridging Science And Nature To Shape Tomorrow — Dr. Nina Siragusa Ph.D. — Merck KGaA, Darmstadt, Germany
#NinaSiragusa #MerckGroup #Darmstadt.
Dr. Nina Siragusa, Ph.D., MBA, is the Strategy, Business, and Data & Digital Lead within the global R&D organization of Merck Healthcare KGaA, Darmstadt, Germany. In this role, she leads strategic projects, manages business operations, and drives digital transformation.
Previously, she served as Chief of Staff to Dr. Laura Matz, Chief Science and Technology Officer at Merck KGaA, Darmstadt, Germany. As part of the Science and Technology Office Leadership Team, she was responsible for fostering cross-sectoral collaboration, innovation, and digitalization across Merck’s three business sectors. She also spearheaded the company’s efforts in Bioconvergence, a multidisciplinary approach that synergizes biology, engineering, data, and digitalization. This initiative promises groundbreaking advancements in healthcare and the life sciences, heralding a new era of scientific collaboration for a healthier, more sustainable future.
Prior to that, Dr. Siragusa contributed to corporate innovation in several leadership roles:
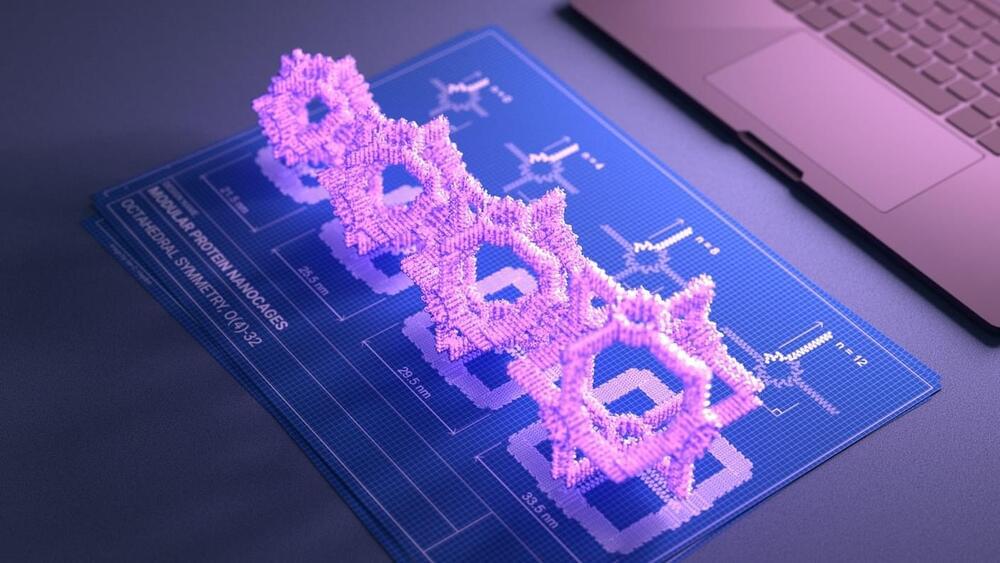
One used AI to dream up a universe of potential CRISPR gene editors. Inspired by large language models—like those that gave birth to ChatGPT—the AI model in the study eventually designed a gene editing system as accurate as existing CRISPR-based tools when tested on cells. Another AI designed circle-shaped proteins that reliably turned stem cells into different blood vessel cell types. Other AI-generated proteins directed protein “junk” into the lysosome, a waste treatment blob filled with acid inside cells that keeps them neat and tidy.
Outside of medicine, AI designed mineral-forming proteins that, if integrated into aquatic microbes, could potentially soak up excess carbon and transform it into limestone. While still early, the technology could tackle climate change with a carbon sink that lasts millions of years.
It seems imagination is the only limit to AI-based protein design. But there are still a few cases that AI can’t yet fully handle. Nature has a comprehensive list, but these stand out.

