The targeted gene used for the edit is conserved across evolution, suggesting the technique could work in more animals than just mice.


When we think about gene editing, the first thing we remember is the designer babies, and that it’s usually called unethical. But actually, gene editing (CRISPR) may be one of the most promising upcoming medical technologies. Learn why in this video.
Check out other videos from this series:
https://www.youtube.com/playlist?list=PLnWSi4zEceYXPCBYXZ9ZEV-9q44ebksoo.
0:00 — Opening scene.
0:20 — Gene editing is promising. Here’s why.
2:35 — Also, it can transform the beauty industry.
3:49 — How does gene editing work?
4:16 — My thoughts on that.
5:16 — End credits.
Text me: [email protected].
Business inquiries: [email protected].
Directed by Valentin Shevtsov.
© Shevtsov Originals, 2021.
#dna #science #medicine.
Jamie Metzl is an author specializing in topics of genetic engineering, biotechnology, and geopolitics. Please support this podcast by checking out our sponsors:
- Mizzen+Main: https://mizzenandmain.com and use code LEX to get $35 off.
- NI: https://www.ni.com/perspectives.
- GiveDirectly: https://givedirectly.org/lex to get gift matched up to $300
- Indeed: https://indeed.com/lex to get $75 credit.
- Blinkist: https://blinkist.com/lex and use code LEX to get 25% off premium.
EPISODE LINKS:
Jamie’s Twitter: https://twitter.com/JamieMetzl.
Jamie’s Website: https://jamiemetzl.com/
Jamie’s lab leak blog post: https://jamiemetzl.com/origins-of-sars-cov-2/
Hacking Darwin (book): https://amzn.to/3lLqLsM
PODCAST INFO:
Podcast website: https://lexfridman.com/podcast.
Apple Podcasts: https://apple.co/2lwqZIr.
Spotify: https://spoti.fi/2nEwCF8
RSS: https://lexfridman.com/feed/podcast/
Full episodes playlist: https://www.youtube.com/playlist?list=PLrAXtmErZgOdP_8GztsuKi9nrraNbKKp4
Clips playlist: https://www.youtube.com/playlist?list=PLrAXtmErZgOeciFP3CBCIEElOJeitOr41
OUTLINE:
0:00 — Introduction.
1:27 — Lab leak.
1:00:01 — Gain-of-function research.
1:09:32 — Anthony Fauci.
1:19:14 — Francis Collins.
1:23:56 — Joe Rogan, Brett Weinstein, and Sam Harris.
1:53:53 — Xi Jinping.
2:08:24 — Patient Zero.
2:21:38 — WHO
2:45:28 — Government transparency.
3:07:28 — Likelihood of a cover-up.
3:09:16 — Future of reproduction.
3:44:55 — Jon Stewart.
3:50:14 — Joe Rogan and Sanjay Gupta.
4:15:19 — Ultramarathons.
4:25:21 — Chocolate.
4:33:34 — One Shared World.
4:48:37 — Hope for the future.
SOCIAL:
- Twitter: https://twitter.com/lexfridman.
- LinkedIn: https://www.linkedin.com/in/lexfridman.
- Facebook: https://www.facebook.com/lexfridman.
- Instagram: https://www.instagram.com/lexfridman.
- Medium: https://medium.com/@lexfridman.
- Reddit: https://reddit.com/r/lexfridman.
- Support on Patreon: https://www.patreon.com/lexfridman
Lex Fridman Podcast full episode: https://www.youtube.com/watch?v=K78jqx9fx2I
Please support this podcast by checking out our sponsors:
- Mizzen+Main: https://mizzenandmain.com and use code LEX to get $35 off.
- NI: https://www.ni.com/perspectives.
- GiveDirectly: https://givedirectly.org/lex to get gift matched up to $300
- Indeed: https://indeed.com/lex to get $75 credit.
- Blinkist: https://blinkist.com/lex and use code LEX to get 25% off premium.
GUEST BIO:
Jamie Metzl is an author specializing in topics of genetic engineering, biotechnology, and geopolitics.
PODCAST INFO:
Podcast website: https://lexfridman.com/podcast.
Apple Podcasts: https://apple.co/2lwqZIr.
Spotify: https://spoti.fi/2nEwCF8
RSS: https://lexfridman.com/feed/podcast/
Full episodes playlist: https://www.youtube.com/playlist?list=PLrAXtmErZgOdP_8GztsuKi9nrraNbKKp4
Clips playlist: https://www.youtube.com/playlist?list=PLrAXtmErZgOeciFP3CBCIEElOJeitOr41
SOCIAL:
- Twitter: https://twitter.com/lexfridman.
- LinkedIn: https://www.linkedin.com/in/lexfridman.
- Facebook: https://www.facebook.com/lexfridman.
- Instagram: https://www.instagram.com/lexfridman.
- Medium: https://medium.com/@lexfridman.
- Reddit: https://reddit.com/r/lexfridman.
- Support on Patreon: https://www.patreon.com/lexfridman
Try Dashlane here: https://www.dashlane.com/isaacarthur.
Get 10% off now with my promo code: isaacarthur.
Genetic Engineering and DNA alteration is an emerging technology with huge ramifications in the future, including potentially altering the DNA of adult humans, not just embryos or plants & animals.
Visit our Website: http://www.isaacarthur.net.
Support us on Patreon: https://www.patreon.com/IsaacArthur.
SFIA Merchandise available: https://www.signil.com/sfia/
Social Media:
Facebook Group: https://www.facebook.com/groups/1583992725237264/
Reddit: https://www.reddit.com/r/IsaacArthur/
Twitter: https://twitter.com/Isaac_A_Arthur on Twitter and RT our future content.
SFIA Discord Server: https://discord.gg/53GAShE
Listen or Download the audio of this episode from Soundcloud: Episode’s Audio-only version:
https://soundcloud.com/isaac-arthur-148927746/dna-manipulati…g-subjects.
Episode’s Narration-only version: https://soundcloud.com/isaac-arthur-148927746/dna-manipulati…ation-only.
Credits:
DNA Manipulation in Living Subjects.
Episode 227; Feb 27, 2020
Writers:
Isaac Arthur.
Editors:
Researchers in China have developed a new three-pronged method to fight liver cancer that shows promise in tests in mice. The technique combines drugs and CRISPR-Cas9 gene editing into lipid nanoparticles, then activates them with ultrasound.
One emerging treatment against cancer is known as sonodynamic therapy (SDT), which involves delivering drugs to the tumor and then activating them with ultrasound pulses. That produces reactive oxygen species (ROS) that can induce oxidative stress on the cancer cells to kill them. Unfortunately, cancer can counter this attack with antioxidant enzymes, reducing the method’s efficiency.
So for the new study, the researchers investigated a way to remove that defense system. The team suspected that they could use CRISPR to switch off a gene called NFE2L2, which cancer cells use to set off their antioxidant defenses. The team packaged both the CRISPR machinery and the ROS-producing drugs into lipid nanoparticles, which could be activated with ultrasound pulses.
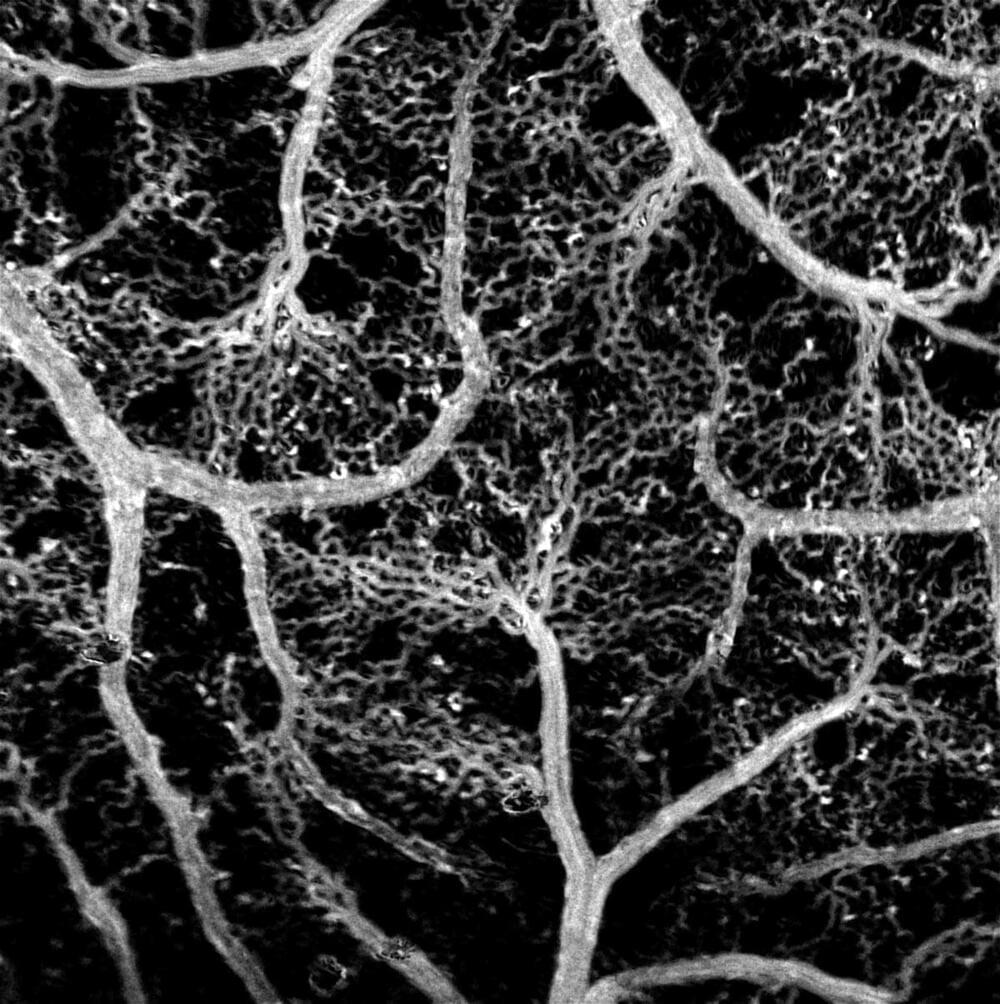
Researchers from the Skolkovo Institute of Science and Technology and Saratov State University have come up with an inexpensive method for visualizing blood flow in the brain. The new technique is so precise it discerns the motions of individual red blood cells — all without the use of toxic dyeing agents or expensive genetic engineering. The study was published in The European Physical Journal Plus.
To understand more about how the brain’s blood supply works, researchers map its blood vessel networks. The resulting visualizations can rely on a variety of methods. One highly precise technique involves injecting fluorescent dyes into the blood flow and detecting the infrared light they emit. The problem with dyes is they are toxic and also may distort mapping results by affecting the vessels. Alternatively, researchers employ genetically modified animals, whose interior lining of blood vessels is engineered to give off light with no foreign substances involved. Both methods are very expensive, though.
Researchers from Skoltech and Saratov State University have devised an inexpensive method for visualizing even the smallest capillaries in the brain. The method — which integrates optical microscopy and image processing — is dye-free and very fine-grained, owing to its ability to detect each and every red blood cell travelling along a blood vessel. Since the number of RBCs in capillaries is not that high, every cell counts, so this is an important advantage over other methods, including dye-free ones.
Transhumanism, briefly explained, means the modification of human beings through technology and engineering. It employs a variety of methods used to cure ailments, or upgrading humans just for the sake of it. Creating people that are smarter, stronger, healthier, or more productive.
It comes with plenty of social and ethical implications and challenges. How will we face this future? Let’s find out today.
Notes and References:
[1] Harari, N.Y. (2017). Homo Deus: A Brief History of Tomorrow. HarperCollins Publishing: New York, NY
[2] Niller, E. (2018, August 10). Why Gene Editing Is the Next Food Revolution. Time. Retrieved from https://www.nationalgeographic.com/environment/article/food-…ne-editing.
[3] Epstein, L.R., Lee, S.S., Miller, M.F., & Lombardi, H.A. (2021). CRISPR, animals, and FDA oversight: Building a path to success. Proceedings of the National Academy of Sciences, 118(22) e2004831117, doi:10.1073/pnas.2004831117
[4] Park, A. (2019, August 6). CRISPR Gene Editing Is Being Tested in Human Patients, and the Results Could Revolutionize Health Care. Time. Retrieved from https://time.com/5642755/crispr-gene-editing-humans/
[5][Neuralink]. (2021, April 8). Monkey MindPong[Video]. YouTube. Retrieved from https://www.youtube.com/watch?v=rsCul1sp4hQ
[6] Ritchie, H., Ortiz-Ospina E. & Roser, M. (2013). Life Expectancy. OurWorldInData.org. Retrieved from https://ourworldindata.org/life-expectancy.
[7] Sinclair, D.A. & LaPlante, M.D. (2019). Lifespan: Why We Age and Why We Don’t Have To. Atria Books: New York, NY.
[8] Kharpal, A. (2017, February 13). Elon Musk: Humans must merge with machines or become irrelevant in AI age. CNBC. https://www.cnbc.com/2017/02/13/elon-musk-humans-merge-machi…obots.html.
[9] Humanity+ —World Transhumanist Association. (n.d.). Retrieved from https://humanityplus.org.
Do you want to learn more about Transhumanism? Check out these sources!
[Kurzgesagt – In a Nutshell]. (2020, December 10). Can You Upload Your Mind & Live Forever?[Video]. YouTube. Retrieved from https://www.youtube.com/watch?v=4b33NTAuF5E.
[Kurzgesagt – In a Nutshell]. (2017, October 20). Why Age? Should We End Aging Forever?[Video]. YouTube. Retrieved from https://www.youtube.com/watch?v=GoJsr4IwCm4&t=1s.
[Kurzgesagt – In a Nutshell]. (2017, November 3). How to Cure Aging – During Your Lifetime?[Video]. YouTube. Retrieved from https://www.youtube.com/watch?v=MjdpR-TY6QU
[BBC Ideas]. (2019, December 31). Transhumanism: Will humans evolve to something smarter? | A-Z of ISMs Episode 20 — BBC Ideas[Video]. YouTube. https://www.youtube.com/watch?v=RVmuU04-X5E
For more cool content follow me on:
▶️ My Film and Gaming YouTube Channel — https://youtube.com/channel/UCpWCw5dVvb4QUGYHRrx4kmw.
👨💻 Twitch — https://twitch.tv/grantnkeegan.
Follow Tomorrow Matters for tech, futurism and environment content:
▶️ YouTube — https://www.youtube.com/channel/UCOKyVx-5zwE_ZwHSJjrjYRQ
📚 My Official Blog — https://grantkeegan.com.
🐦 Twitter — https://twitter.com/TomorrowM4tters.
📷 Instagram — https://instagram.com/tomorrow_matters_

A scientist who loves to write, can do it well, and can share the excitement of the scientific pursuit is incredibly rare. Kevin Peter Hand 0, Deputy Project Scientist, Europa and Director of the JPL Ocean Worlds Lab is that rare person who can do all these things. In his incredible book Alien Oceans: The Search for Life in the Depths of Space 0, he explains that “We know that the laws of physics, the principles of chemistry, and the principles of geology all work beyond Earth. We’ve explored other worlds and observed that these sciences are universal. When it comes to biology, however, we have yet to make that leap.”
If you want to learn about how the intersection of numerous areas of science are helping inform our understanding of the oceans, space, and ourselves, Alien Oceans is by far one of the most clearly written books on the topic. As Kevin notes, he wrote the book he wishes he could have read in college. Kevin will teach you and inspire you and explain complicated scientific topics in ways nearly anyone can understand. Not only is it a book about his areas of expertise, it is also a wonderful window into the way scientists and engineers think about solving real world problems and applying basic knowledge. For example, Kevin notes in this interview that “Making measurements is where the creativity of science meets the hard reality of engineering.” I read a lot of books on science written for a broad audience, and this book, by far is among the very best I have ever read. More than anything else what came through in Kevin’s writing is excitement about finding out what is true.
What inspired you to write this book?