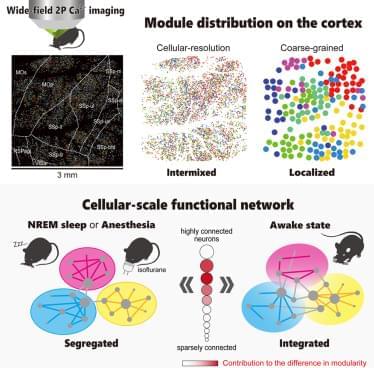
The common neural mechanisms underlying the reduction of consciousness during sleep and anesthesia remain unclear. Previous studies have examined changes in network structure by only using recordings with limited spatial resolution, which has hindered the investigation of the critical spatial scales for the reduction of consciousness. To address this issue, we recorded calcium signals from approximately 10,000 neurons across multiple cortical regions in awake, sleeping, and anesthetized mice and compared network structure at different spatial scales by leveraging single-cell resolution and wide-field two-photon microscopy. At the single-cell scale, both sleep and anesthesia exhibit higher network modularity than an awake state, indicating a segregated network, but modules are spatially intermixed in all three states.