Researchers know how to make precise genetic changes within the genomes of crops, but the transformed cells often refuse to grow into plants. One team has devised a new solution.
Scientists who want to improve crops face a dilemma: it can be difficult to grow plants from cells after you’ve tweaked their genomes.
A new tool helps ease this process by coaxing the transformed cells, including those modified with the gene-editing system CRISPR-Cas9, to regenerate new plants. Howard Hughes Medical Institute Research Specialist Juan M. Debernardi and Investigator Jorge Dubcovsky, together with David Tricoli at the University of California, Davis Plant Transformation Facility, Javier Palatnik from Argentina, and colleagues at the John Innes Centre, collaborated on the work. The team reports the technology, developed in wheat and tested in other crops, October 12, 2020, in the journal Nature Biotechnology.
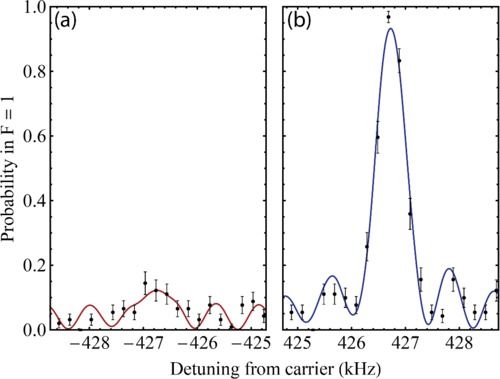
“The problem is that transforming a plant is still an art,” Dubcovsky says. The success rate is often low – depending on the crop being modified, 100 attempts may yield only a handful of green shoots that can turn into full-grown plants. The rest fail to produce new plants and die. Now, however, “we have reduced this barrier,” says Dubcovsky, a plant geneticist at UC Davis. Using two genes that already control development in many plants, his team dramatically increased the formation of shoots in modified wheat, rice, citrus, and other crops.