A new type of catalyst—a material that speeds up chemical reactions—that could make the production of clean hydrogen fuel more efficient and long-lasting has been developed by a team led by City University of Hong Kong, including researchers from Hong Kong, mainland China, and Japan.
This breakthrough uses high-density single atoms of iridium (a rare metal) to greatly improve the process of splitting water into hydrogen and oxygen, which is key to renewable energy technologies like hydrogen fuel cells and large-scale energy storage.
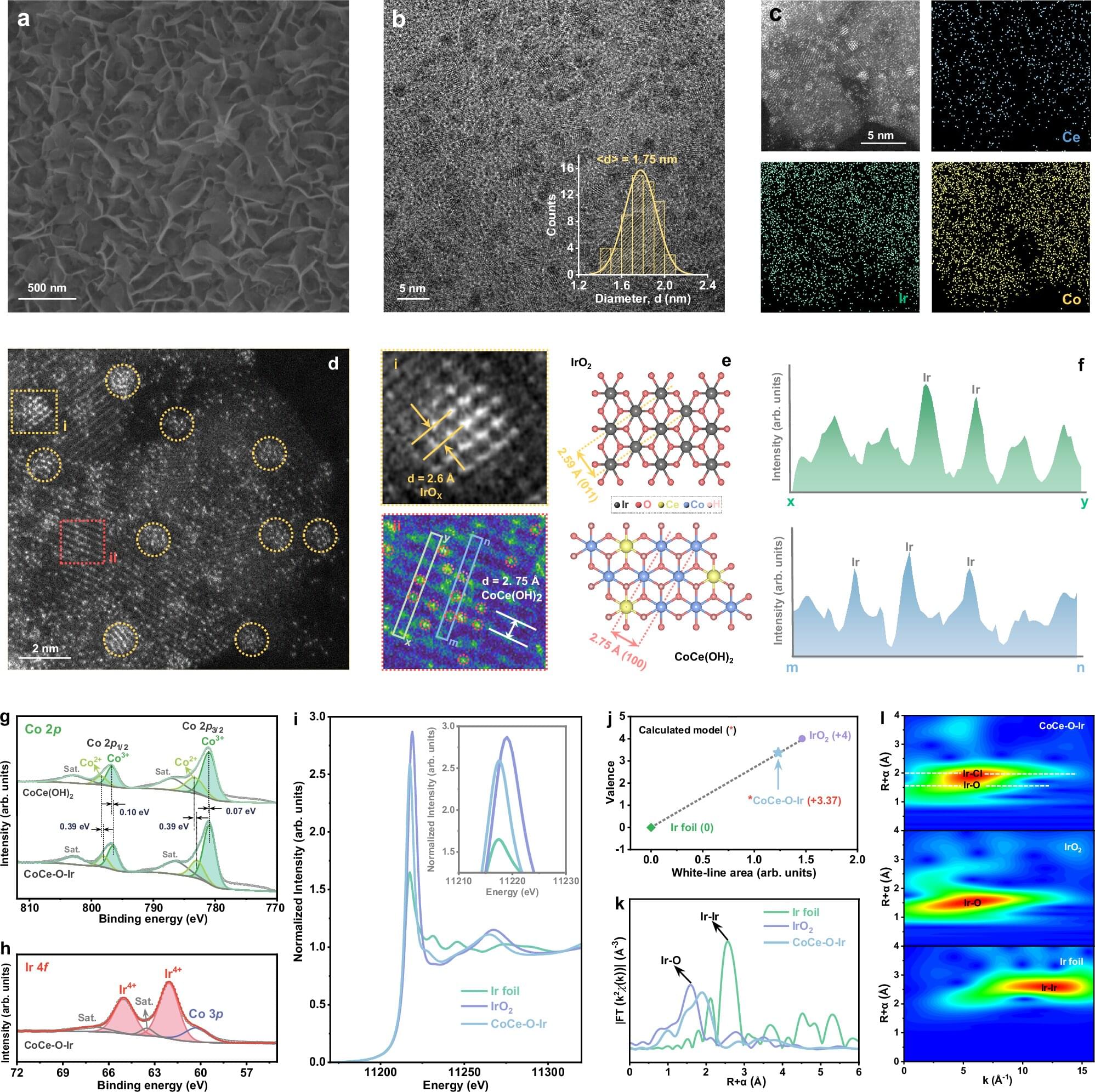
The researchers created a highly stable and active catalyst by placing single iridium atoms on ultra-thin sheets made of cobalt and cerium compounds. Called CoCe–O–IrSA, the final product performs exceptionally well in the water-splitting process. It requires very little extra energy (just 187 mV of overpotential at 100 mA cm-2) to drive the oxygen evolution reaction at a high rate, and it stays stable for more than 1,000 hours under demanding conditions.