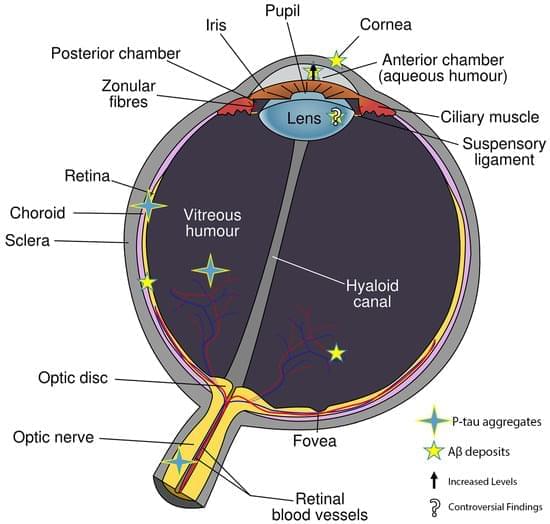
Hypertriglyceridemia-associated acute pancreatitis (HLAP) is a severe gastrointestinal condition characterized by an increased risk of multiple organ dysfunction and elevated mortality. Intestinal microbiota, often described as the second human genome, plays a key role in maintaining gastrointestinal and systemic homeostasis. Among its various metabolites, short-chain fatty acids (SCFAs) are particularly abundant and functionally significant. Current evidence indicates a strong relationship between SCFAs and the pathogenesis and progression of HLAP. SCFAs contribute to the restoration of intestinal homeostasis by modulating the composition of gut microbiota, enhancing the integrity of the intestinal epithelial barrier, and regulating mucosal immune responses. Furthermore, SCFAs attenuate systemic inflammatory responses, promote pancreatic tissue repair, and reduce the risk of multiple organ dysfunction. These protective effects indicate that SCFAs represent a promising therapeutic target for gut-centered interventions in HLAP. This review summarizes the changes in intestinal microbiota and SCFA levels following HLAP onset, elucidates the underlying mechanisms by which SCFAs exert protective effects, and evaluates their potential therapeutic applications, thereby providing a theoretical basis for the development of gut-targeted strategies in the management of HLAP.
Acute pancreatitis (AP) is characterized by acute inflammation and cellular injury within the pancreas and is recognized as a common cause of acute abdominal disorders. With improvements in living standards and shifts in dietary habits, the incidence of hypertriglyceridemia-associated acute pancreatitis (HLAP) has significantly increased, surpassing alcoholic pancreatitis to become the second leading cause of AP (Chinese Pancreatic Surgery Association, and Chinese Society of Surgery, Chinese Medical Association, 2021). Additionally, HLAP is increasingly observed in younger adults and is associated with severe clinical presentations, including a higher incidence of complications such as acute respiratory distress syndrome (ARDS), acute kidney injury (AKI), and multiple organ dysfunction syndrome (MODS) (Li et al., 2018).