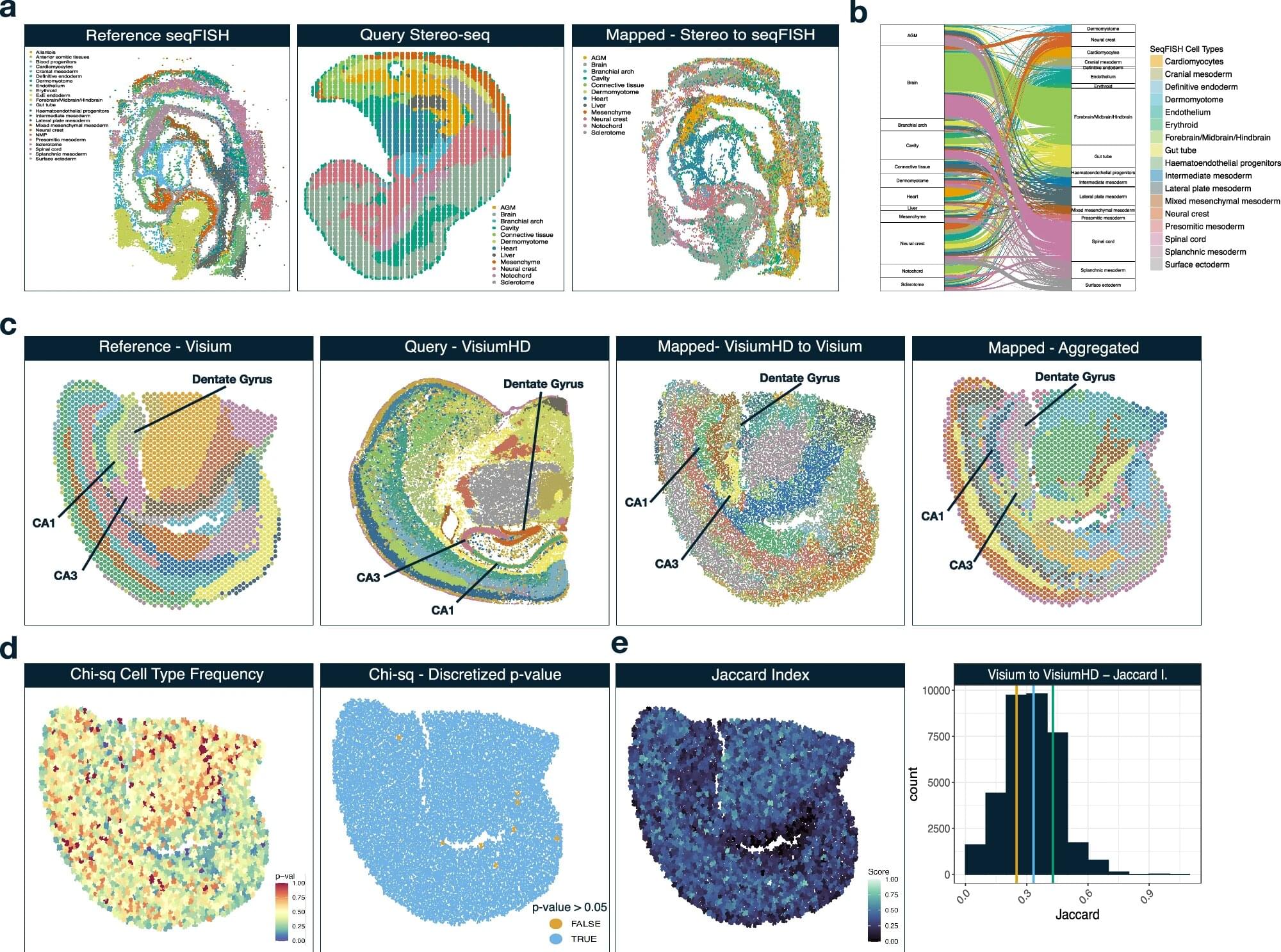
Researchers at VCU Massey Comprehensive Cancer Center have developed a new computational tool called Vesalius, which could help clinicians understand the complex relationships between cancer cells and their surrounding cells, leading to potential discoveries regarding the development of hard-to-treat cancers.
Findings from a new study, published in Nature Communications, could help guide the identification of predictive biomarkers for multiple cancers and better inform the effectiveness of different treatment options based on individuals’ specific type of disease.
Rajan Gogna, Ph.D., member of the Developmental Therapeutics research program at Massey and assistant professor in the VCU School of Medicine’s Department of Human and Molecular Genetics, and a team of collaborators were driven by the goal of interpreting extensive amounts of data in a meaningful way.