Table of contents introduction: the limits of classical scaling the origins of machine…
Unitree Robotics introduces a revolutionary open-source data set allowing humanoid robots to move more naturally.
Now millions of developers are building with Gemini. And it’s helping us reimagine all of our products — including all 7 of them with 2 billion users — and to create new ones. NotebookLM is a great example of what multimodality and long context can enable for people, and why it’s loved by so many.
Over the last year, we have been investing in developing more agentic models, meaning they can understand more about the world around you, think multiple steps ahead, and take action on your behalf, with your supervision.
Today we’re excited to launch our next era of models built for this new agentic era: introducing Gemini 2.0, our most capable model yet. With new advances in multimodality — like native image and audio output — and native tool use, it will enable us to build new AI agents that bring us closer to our vision of a universal assistant.
NVIDIA and TSMC have developed a silicon photonics-based chip prototype, according to a report in the Taiwanese press. TSMC is the world’s leading contract chip manufacturer, and with Intel’s troubles, it has also established itself as the most advanced chip manufacturer on the planet. Silicon photonics is an emerging chip manufacturing technology that blends photonic circuits with traditional circuits to overcome physical limitations with semiconductor fabrication. According to the report, the prototype was developed late last year, with NVIDIA and TSMC also working on optical packaging technologies to improve AI chip performance.
NVIDIA & TSMC Are Working On Advanced Packaging Technologies, Says Report
TSMC’s latest chip manufacturing technology, the 2-nanometer node, is believed to have a minimum gate and metal pitches of 45 and 20 nanometers, respectively. In semiconductor fabrication, a gate pitch measures the distance between two gates on a chip, while a metal pitch measures the distance between two metal interconnects. A gate controls the flow of electrons on a transistor, while an interconnect ensures inter-transistor communication on a chip.
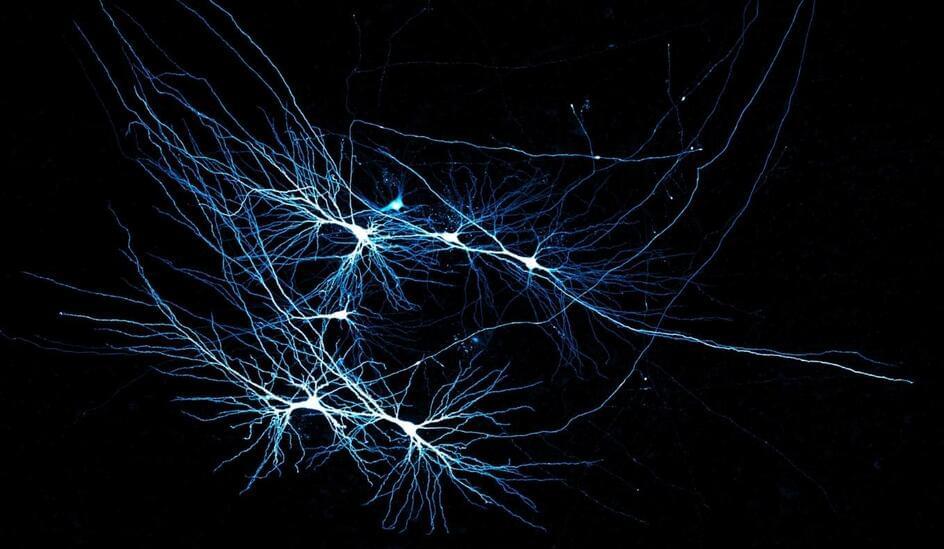
Our brain’s memory center bears a sleek design.
A peek into living tissue from human hippocampi, a brain region crucial for memory and learning, revealed relatively few cell-to-cell connections for the vast number of nerve cells. But signals sent via those sparse connections proved extremely reliable and precise, researchers report December 11 in Cell.
One seahorse-shaped hippocampus sits deep within each hemisphere of the mammalian brain. In each hippocampus’s CA3 area, humans have about 1.7 million nerve cells called pyramidal cells. This subregion is thought to be the most internally connected part of the brain in mammals.
Engineers from the University of Massachusetts Amherst in the US are developing a device that can harvest humidity in the air to create clean electricity.
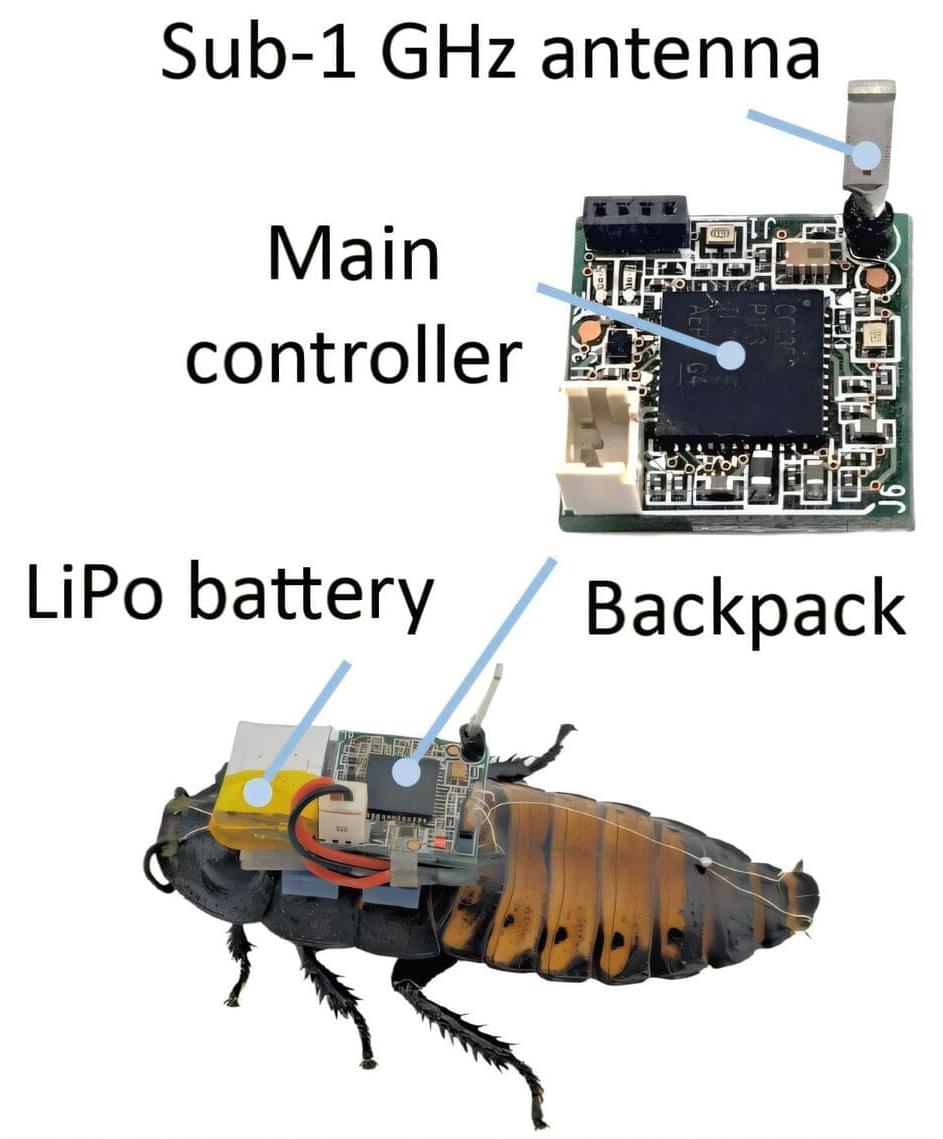
Scientists have developed an advanced swarm navigation algorithm for cyborg insects that prevents them from becoming stuck while navigating challenging terrain.
Published in Nature Communications, the new algorithm represents a significant advance in swarm robotics. It could pave the way for applications in disaster relief, search-and-rescue missions, and infrastructure inspection.
Cyborg insects are real insects equipped with tiny electronic devices on their backs—consisting of various sensors like optical and infrared cameras, a battery, and an antenna for communication—that allow their movements to be remotely controlled for specific tasks.
Why it matters
The new study explains a longstanding puzzle in medicine: why do some people who’ve inherited a disease-causing mutation experience fewer symptoms than others with the same mutation? “In many diseases, we’ll see that 90% of people who carry a mutation are sick, but 10% who carry the mutation don’t get sick at all,” says Bogunovic, a scientist who studies children with rare immunological disorders at Columbia University Irving Medical Center.
Enlisting an international team of collaborators, the researchers looked at several families with different genetic disorders affecting their immune systems. In each case, the disease-causing copy was more likely to be active in sick patients and suppressed in healthy relatives who had inherited the same genes.
What if the secret to slowing down aging was hiding in our brains? A groundbreaking study by researchers at the Allen Institute for Brain Science in Seattle, published in Nature in January 2025, may have uncovered some exciting clues. Using cutting-edge technology, the team analyzed over 1.2 million brain cells from young and aged mice to understand how they change with time. They found that certain cells become inflamed, while others lose critical functions, and all eyes are now on the hypothalamus as a key player in the aging process. These findings deepen our understanding of aging and could pave the way for treatments that keep our brains younger for longer.