Fungi isolated from rotting hardwood trees can break down sheets of low-density polyethylene, one of the most abundant plastics on Earth.
By Chen Ly

Fungi isolated from rotting hardwood trees can break down sheets of low-density polyethylene, one of the most abundant plastics on Earth.
By Chen Ly

GitHub Copilot is getting an upgrade with an improved AI model and enhanced contextual filtering, resulting in faster and more tailored code suggestions for developers.
The new AI model delivers a 13% improvement in latency, while enhanced contextual filtering delivers a 6% relative improvement in code acceptance. These improvements are coming to GitHub Copilot for Individuals and GitHub Copilot for Business.
According to Github, the new model was developed together with OpenAI and Azure AI, and the 13% improvement in latency means that GitHub Copilot generates code suggestions for developers faster than ever before, promising a significant increase in overall productivity.

Nine brains, blue blood, instant camouflage: It’s no surprise that octopuses capture our interest and our imaginations. Science-fiction creators, in particular, have been inspired by these tentacled creatures.
An octopus’s remarkable intelligence makes it a unique subject for marine biologists and neuroscientists as well. Research has revealed the brain power of the octopus allows it to unscrew a jar or navigate a maze. But, like many children, the octopus also develops an impish tendency to push the boundaries of behavior. Several aquariums have found octopuses memorizing guard schedules to sneak into nearby tanks to steal fish; meanwhile, marine biologists have discovered that wild octopuses will punch fish … for no apparent reason.
According to Dr. Jennifer Maher, a professor at the University of Lethbridge in Canada, there are a “number of [different] types of learning [for octopuses]: cognitive tasks like tool use, memory of complex operations for future use, and observational learning.”

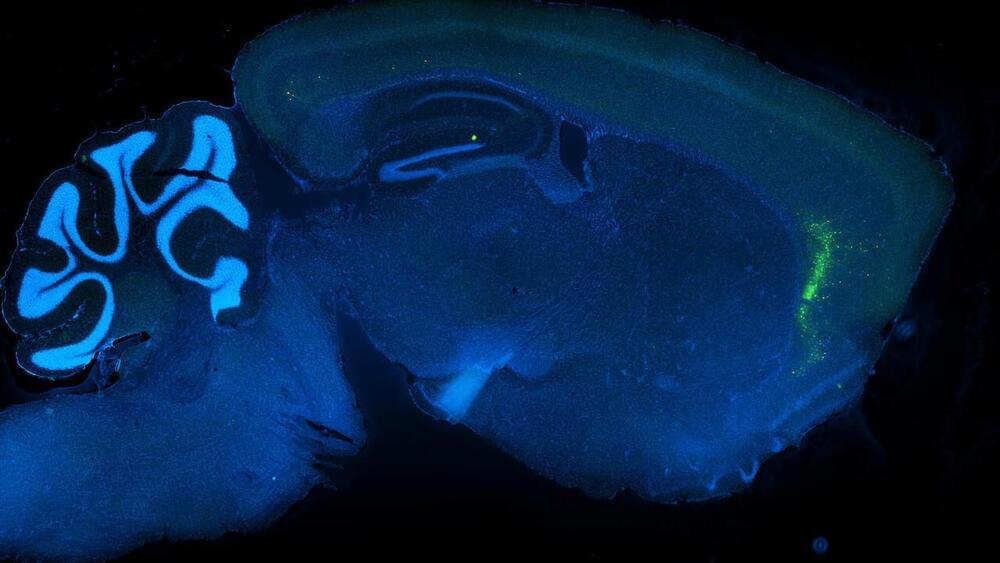
Recent collaborative research conducted by scientists in the United States and China unveils the mechanism through which a fertilized egg cell, also known as a zygote, triggers a ‘reset’, enabling the newly formed embryo can develop according to its own genetic program. The study was recently published in the journal Nature.
It has been known for some time that the genome of a newly fertilized egg cell is inactive and has to be woken up, said Richard Schultz, research professor at the University of California, Davis, School of Veterinary Medicine and a corresponding author on the paper. This step is called zygote genome activation.
“For the embryo to develop, the oocyte/egg has to lose its identity and does so by making new stuff,” Schultz said. “We now know the first steps in how this transition occurs.”


Two meteor showers, the Delta Aquariids and Alpha Capricornids, are both expected to peak during the evenings of July 30 and 31.
They will have to compete with a bright moon that’s 95% full, but the Alpha Capricornids may still produce some scintillating fireballs that could outshine the moon.
The Delta Aquariids, also called the Southern Delta Aquariids, will favor skywatchers in the Southern Hemisphere, although they will still be visible in the Northern Hemisphere (especially across the southern US) but lower on the horizon, according to EarthSky.
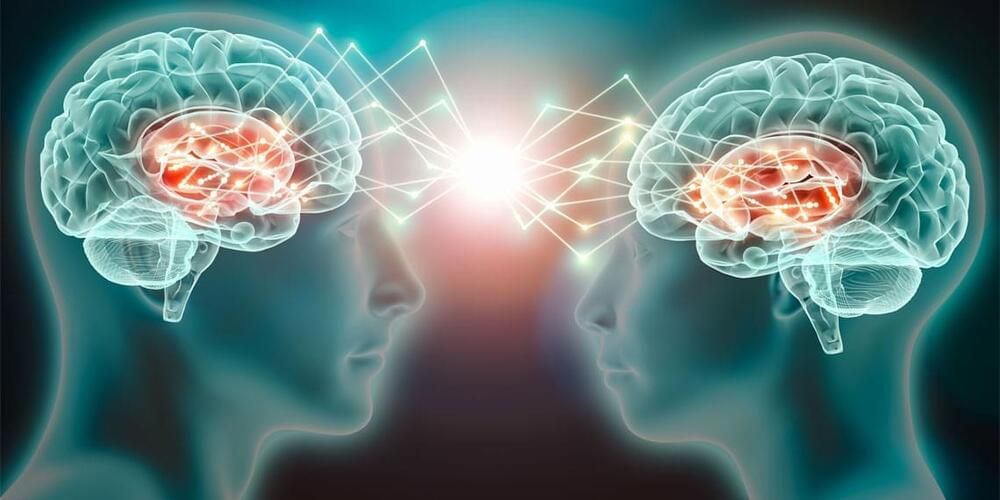
Just by observing the natural behavior of someone we know well, our brain activity can start to sync up with theirs, according to new research published in NeuroImage. The findings shed light on the fascinating interplay between social behavior and brain activity.
Successful social interaction depends on our ability to exchange information with others and continuously update our understanding of their inner states and actions. The authors of the new study sought to better understand the role of a phenomenon called interpersonal neural synchrony (INS) – the alignment of brain activities between people who are interacting.
Previous studies have supported the idea that INS can predict the success of social interactions. However, most research on INS has focused on structured social tasks, trying to establish a relationship between INS and social behavior. What has been less clear is how INS originates or what triggers it.
