Three PCIe IDE flaws in PCIe 5.0+ allow stale or incorrect data handling, fixed by updated PCIe 6.0 guidance.



Structural insights into PRC2 function in development and disease.
Polycomb repressive complex 2 (PRC2) is a central epigenetic regulator of developmental gene repression that displays remarkable complexity arising from multiple molecular layers.
Enzyme catalysis and chromatin targeting form the basis of the common and distinct functions of PRC2.1 and PRC2.2, serving as focal points in the cellular regulation of PRC2 activity under both physiological and pathological contexts.
Structural biology has begun to clarify the molecular mechanisms underlying key functions of PRC2 and uncover new modes of regulation, with much still remaining to be understood about the elaborate system of PRC2-mediated gene control. https://sciencemission.com/PRC2-function-in-development-and-disease
Polycomb repressive complex 2 (PRC2) is a key epigenetic enzyme complex that mediates developmental gene repression mainly by depositing the repressive H3K27me3 histone mark. PRC2 operates through its distinct forms, PRC2.1 and PRC2.2, each defined by unique accessory subunits, with additional complexity introduced by other molecular variants such as developmentally regulated homologs and isoforms. PRC2 function is primarily dictated by its enzymatic activity and chromatin recruitment, both of which are rigorously controlled during development and can be dysregulated by disease-associated mutations and oncoproteins. Structural biology has begun to provide important mechanistic insights into various aspects of PRC2 assembly, catalysis, chromatin targeting, and cellular regulation at atomic resolution, addressing several longstanding questions about the Polycomb repression system.

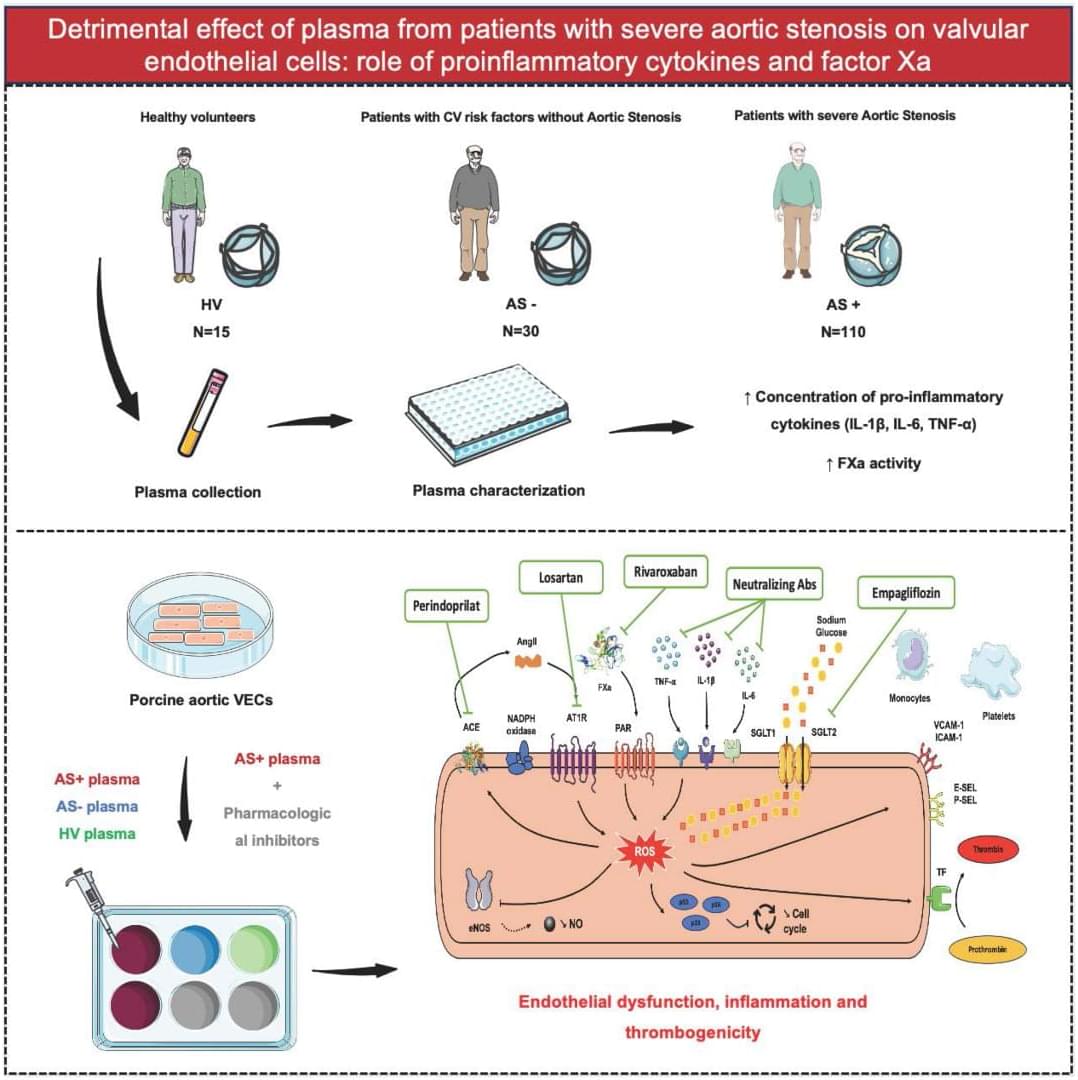
Severe AS plasma is pro-inflammatory and pro-thrombotic: it drives oxidative stress + endothelial dysfunction + monocyte/platelet adhesion in VECs. Multiple drug classes blunted these effects. @A_Trimaille @adrien_carmona @BMarchandot
Aortic stenosis (AS) is the most prevalent valvular heart disease in developed countries.1 Despite its widespread occurrence, no medical treatment has been validated to prevent the thickening of the aortic valve and the reduction in its opening area. The only available therapeutic option to date remains surgical or transcatheter aortic valve replacement once the severity criteria are met.2, 3 A comprehensive understanding of AS pathophysiology is therefore essential for identifying new therapeutic targets.
Initially thought to be a passive degenerative process, AS is now recognized as an active, multifaceted condition involving numerous cellular and molecular contributors.1, 4 The progression of AS follows a chronological sequence, beginning with the damage and dysfunction of valvular endothelial cells (VECs) due to biomechanical forces acting on the aortic valve. This damage promotes intravalvular inflammation and neoangiogenesis, followed by myofibroblastic and osteoblastic differentiation of valvular interstitial cells. Additionally, several lines of evidence suggest a bidirectional interaction between the pathomechanisms of AS and various components of the hemostatic system, including platelets, tissue factor, thrombin, von Willebrand factor, and extracellular vesicles.4, 5, 6
Given the pivotal role of VECs in maintaining valvular homeostasis under physiological conditions, and their early involvement in AS pathogenesis, they are considered key actors in the disease process. Although biomechanical factors have been identified as primary triggers of VEC dysfunction in the early stage of AS,4 the molecular mechanisms underlying the interaction between plasma from patients with AS and aortic VECs remain unclear. Because plasma contains various biological effectors potentially contributing to endothelial cell dysfunction, including proinflammatory cytokines and hemostatic factors,4, 7 this study aimed to investigate whether plasma from patients with AS induces oxidative stress and contributes to VEC dysfunction.


Dr. Somrita Banerjee: “This is the first time AI has been used to help control a robot on the ISS. It shows that robots can move faster and more efficiently without sacrificing safety, which is essential for future missions where humans won’t always be able to guide them.”
How can an AI robot help improve human space exploration? This is what a recent study presented at the 2025 International Conference on Space Robotics hopes to address as a team of researchers investigated new methods for enhancing AI robots in space. This study has the potential to help scientists develop new methods for enhancing human-robotic relationships, specifically as humanity begins settling on the Moon and eventually Mars.
For the study, the researchers examined how a technique called machine learning-based warm starts could be used to improve robot autonomy. To accomplish this, the researchers launched the Astrobee free-flying robot to the International Space Station (ISS), where its algorithm was tested floating around the ISS in microgravity. The goal of the study was to ascertain if Astrobee could navigate its way around the ISS without the need for human intervention, relying only on its algorithm to determine safely traversing the ISS. In the end, the researchers found that Astrobee successfully navigated the tight terrain of the ISS with limited need for human intervention.


The immune system uses regulatory T cells to dampen inflammatory responses. However, the mechanism that led to these tolerant cells was unclear.
Now, a team of researchers showed that the trigger lies in a protein used in red blood cell production.
Read more.
Erythropoietin, the protein that drives red blood cell formation, also induces tolerance in dendritic cells, leading to the development of regulatory T cells.

What if your conscious experiences were not just the chatter of neurons, but were connected to the hum of the universe? In a paper published in Frontiers in Human Neuroscience, I present new evidence indicating that conscious states may arise from the brain’s capacity to resonate with the quantum vacuum—the zero-point field that permeates all of space.
More specifically, I argue that macroscopic quantum effects are at play inside our heads. This insight results from a synthesis of brain architectural and neurophysiological findings supplemented with quantitative model calculations. The novel synthesis suggests that the brain’s basic functional building blocks, cortical microcolumns, couple directly to the zero-point field, igniting the complex dynamics characteristic of conscious processes.

More hints that the Singularity really has begun: and more importantly: https://arstechnica.com/space/2025/12/after-years-of-resisti…ublic-why/
The second article is how Elon is going to have SpaceX go public at $1.5 trillion so he has more money to put into AI. Of course, Elon is not the only one putting money into AI and $1 trillion will be spent on AI data centers next year.
Due to rising prices from the “AI” bubble, Samsung Semiconductor reportedly refused a RAM order for new Galaxy phones from Samsung Electronics.