Staging is the process of determining how much cancer is within the body (tumor size) and if it has spread. Learn about the TNM system and other ways that stage is described.

A recent study found increased cardiac arrhythmia risk to stay long term in individuals with epilepsy, specially in people who use carbamazepine and valproic acid. The findings of the study were published in European Heart Journal.
Using UK Biobank data, the research also explores the potential roles of genetics and antiseizure medications (ASMs) in this complex relationship. Encompassing 329,432 participants, the study included 2,699 with epilepsy, was initiated between 2006 and 2010. Using advanced statistical techniques like Cox proportional hazards models and competing risk models, the researchers aimed to determine the association between epilepsy history and the incidence of cardiac arrhythmias over an extended period.
Individuals with epilepsy displayed a staggering 36% increased risk of experiencing any form of cardiac arrhythmia compared to those without the condition. This risk extended to specific arrhythmia subtypes, including atrial fibrillation, where a 26% increased risk was identified. More alarmingly, the risk of other cardiac arrhythmias was found to be 56% higher in epilepsy patients.
How would it feel to control objects with your mind? Or hear colors? Or maybe even live forever? Well, if you want to find out, all you have to do is become a cyborg. How would being part machine affect us? Would it cause a greater divide between the rich and the poor? And is this the next step in human evolution?
Transcript and sources: https://whatifshow.com/what-if-we-become-cyborgs/
Can you translate this episode into another language? Add subtitles and we will link your YouTube channel in the description: https://www.youtube.com/timedtext_video?v=t6Enng4xZUs.
Watch more what-if scenarios:
Planet Earth: http://bit.ly/YT-what-if-Earth.
The Cosmos: http://bit.ly/YT-what-if-Cosmos.
Technology: http://bit.ly/YT-what-if-Technology.
Your Body: http://bit.ly/YT-what-if-Body.
Humanity: http://bit.ly/YT-what-if-Humanity.
T-shirts and merch: http://bit.ly/whatifstore.
Suggest an episode: http://bit.ly/suggest-whatif.
Newsletter: http://bit.ly/whatif-newsletter.
Feedback and inquiries: https://underknown.com/contact/
Transhumanism — advocates strongly for humans to develop and make widely available sophisticated technologies that enhance human physiology and intellect greatly. In layman’s terms, transhumanists would like for human beings to become cyborgs; cybernetic organisms.
As such, transhumanist concepts feature greatly in science fiction. Cyborgs are commonly seen in all forms of science fiction media…
Concepts of transhumanism and the wish to improve human physiology beyond normal bounds comes from an age-old human desire. That desire is the desire for immortality. Such wishes have been expressed in literature and rhetoric as far back as the early Bronze Age.
It would still take quite some time after the industrial revolution for early transhumanist thinking to develop. Advanced technological growth could eventually allow humans to accomplish much more than a fully fit natural born and grown human can.
As of 2020, transhumanists are playing an established role in global politics in the west, with many of them even being elected to legislature within their respective states. For now, transhumanism just seems like a concept that may or may not be realized practically in the distant future, far beyond our lifetimes.
While being engrossed in our fantasies about the possibilities that may be brought about by cybernetic enhancements to the human body, we tend to forget the important minor details that are very easy to miss. In case of a parts malfunction leading to injury of other people, who is liable? The wearer or the manufacturer of the part?

Get the Breakthrough Tools You Need to Reverse Your Symptoms & Feel 17 Years Younger 83% Discount Expires Soon OWN THE SERIES Get Lifetime Access To The Entire 9-Part Series… PLUS 96 Full-length Expert Interviews, 22 Full-length Elder Interviews, Transcripts, MP3s, Action Plan… AND 10 Bonuses Valued At $1,015 – Yours FREE! Normally $447 Right […].
Nominations are now open for the 3D Printing Industry Awards 2023. Who are the leaders in 3D printing? Find out on November 30th when the winners across twenty categories will be announced during a London-based live awards ceremony.
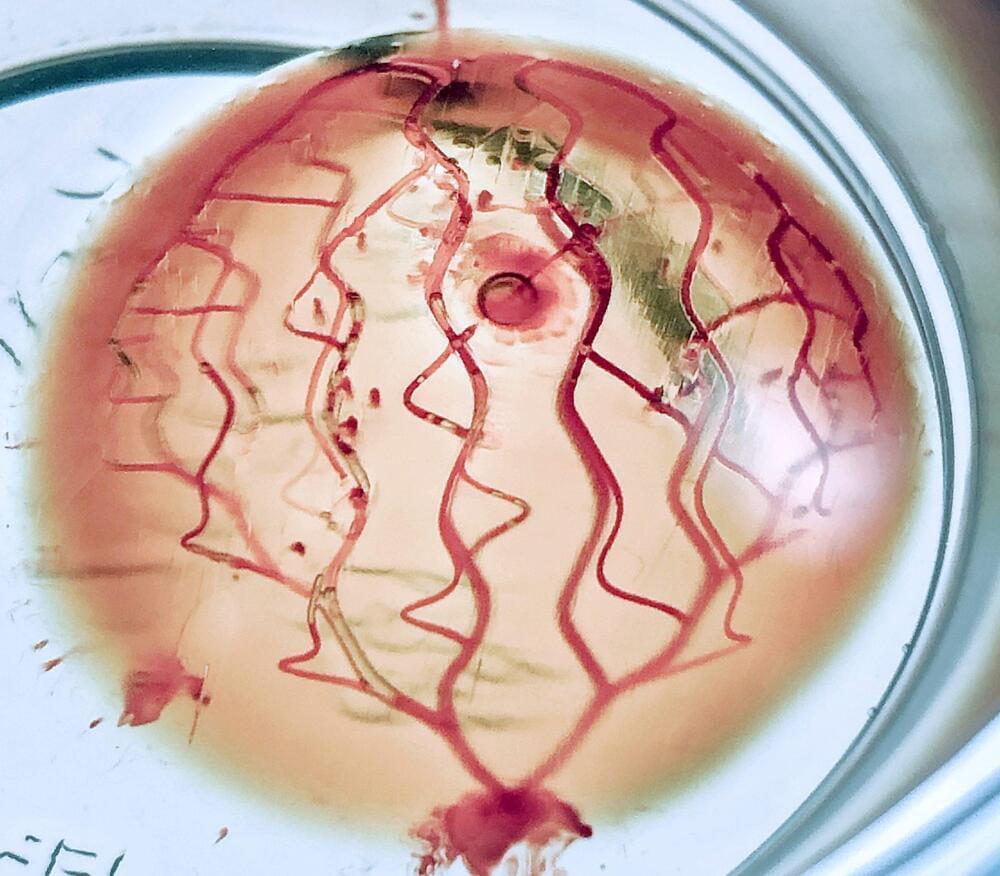
A team of scientists from the University of Sydney and the Children’s Medical Research Institute (CMRI) at Westmead have leveraged 3D photolithographic printing to fabricate functional human tissues that accurately mimic an organ’s architecture.
The researchers utilized bioengineering and cell culture techniques to instruct stem cells derived from blood cells and skin cells to become specialized. These specialized cells can then form organ-like structures.
On a drizzly afternoon, Yan Zhao pointed to the trees visible from his campus window.
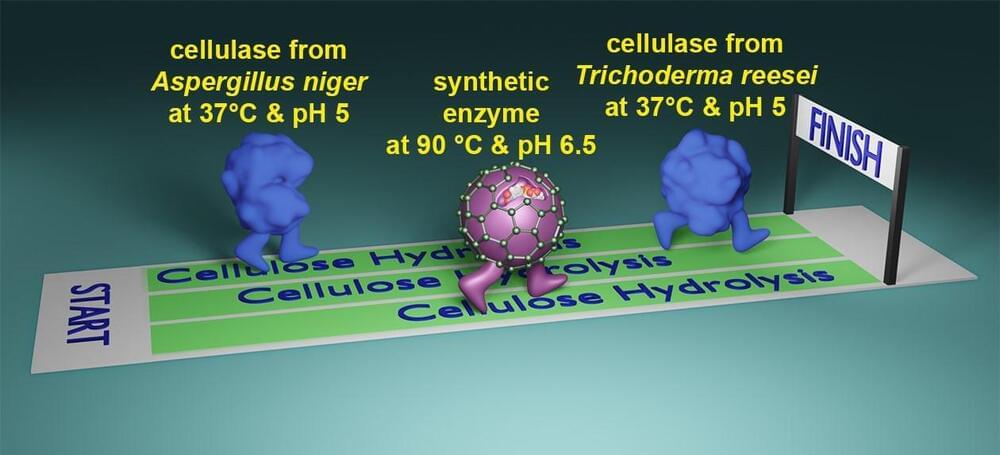
As a chemistry professor at Iowa State University, he is pioneering the creation of novel synthetic catalysts that break down cellulose, the plant fibers responsible for the trees’ height and strength.
“Cellulose is built to last – a tree doesn’t just disappear after rain,” Zhao said. “Cellulose is a huge challenge to break down.”