Fractyl Health is developing a shot that would program the body to make more of the GLP-1 hormone naturally, a risky bet that it can provide a longer-lasting benefit than blockbuster weight-loss drugs on the market.




Elon Musk recently announced that Neuralink, his company aiming to revolutionize brain-computer interfaces (BCIs), has successfully implanted a brain chip in a human for the first time. The implantation of the device, called “the Link,” represents a leap forward in the realm of BCIs, which record and decode brain activity, that may allow for new innovations in health care, communication, and cognitive abilities.
Though limited information on the technology is available and Neuralink’s claims have not been independently verified, here’s a look at the Link, its functionality, and the potential implications of this groundbreaking innovation.
Is interstellar travel doomed to remain in the realm of science fiction? Sticking to near future space propulsion only, how close can we get to the speed of light?
This video looks at the current spacecraft speed records with Apollo 10 holding the record for the fastest manned spacecraft, New Horizons probe for the fastest Earth escape velocity and the Helios probes for the fastest heliocentric velocity. But Solar Probe Plus will beat that when it launches in 2018. While Voyager 1 doesn’t set any speed records, it was the first spacecraft to leave the solar system, so therefore the fastest solar system escape velocity by default.
For beating these speeds, this video explores what is possible in the near future only, so no antimatter, Alcubierre drives (warp), ramjets, etc… The EM drive is left out until it’s proven with actual reproducible results in space.
Project Daedalus and the updated Project Icarus represent sound concepts for fusion spacecraft. IKAROS was the first successful demonstration of solar sail technology but hopefully the planetary society is not far behind with their LightSail cubsat (not covered in this video).
But what appears to have the most potential to reach the nearest star to our own, Proxima Centauri and it’s newly discovered planet Proxima b is Breakthrough Starshot. Thousands of super lightweight laser sail nanocraft will be launched into space then the light beamer, a ground based laser array will propel these spacecraft to 20% light speed within minutes.
All sources used in researching this video are listed in the end credits.
24K views 3 years ago #bengoertzel #singularity #meditation
Mathematica creator Stephen Wolfram has spent nearly 50 years arguing that simple computational rules underlie everything from animal patterns to the laws of physics. In his 2023 TED talk, he makes the case that computation isn’t just a useful way to model the world — it’s the fundamental operating system of reality itself.
Wolfram introduces “the ruliad,” an abstract concept encompassing all possible computational processes. Space and matter, he argues, consist of discrete elements governed by simple rules. Gravity and quantum mechanics emerge from the same computational framework. The laws of physics themselves are observer-dependent, arising from our limited perspective within an infinite computational structure.
On AI, Wolfram sees large language models as demonstrating deep connections between semantic grammar and computational thinking. The Wolfram Language, he claims, bridges human conceptualization and computational power, letting people operationalize ideas directly — what he calls a “superpower” for thinking and creation.

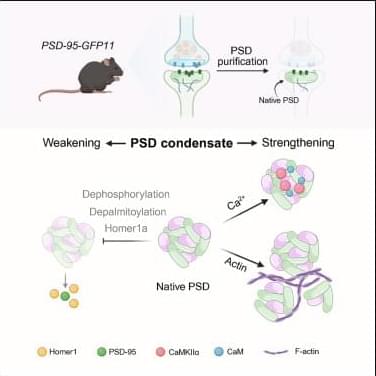
To obtain direct evidence supporting the theory that the postsynaptic density (PSD) in neuronal synapses is formed via phase separation, Chen et al. purified and characterized the native PSD from the mouse brain. Their results demonstrate that the native PSD has characteristic features of biological condensates formed via phase separation.
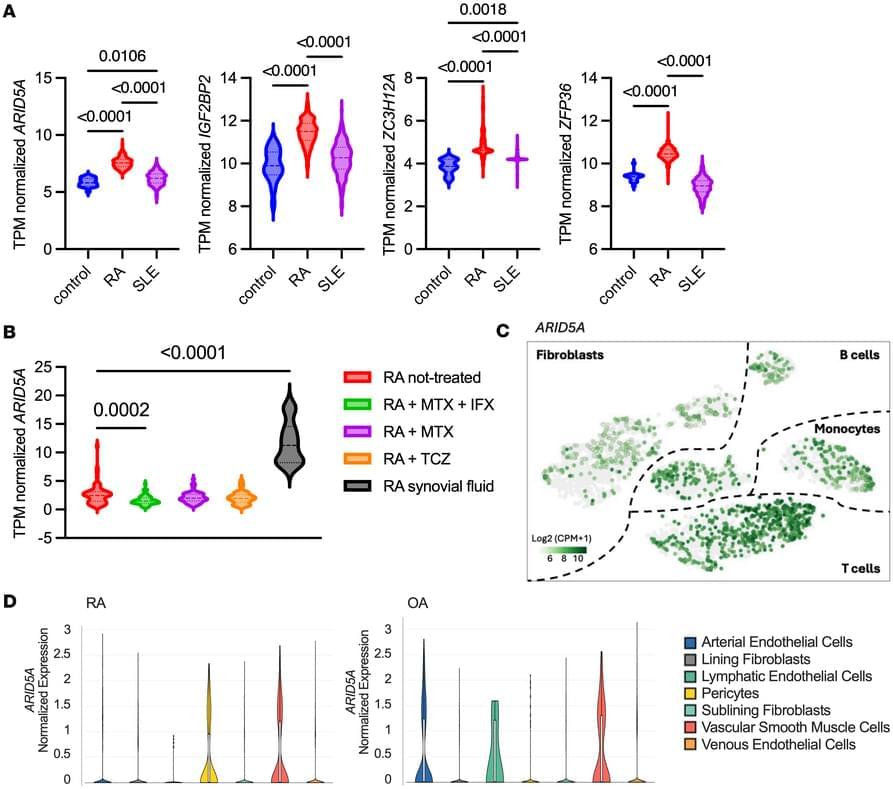
Here, Sarah Gaffen & team report on the noncanonical RNA binding protein Arid5a as an activator of TNF signaling that is elevated in human RA tissues. And in mice… its loss results in resistance to collagen-induced arthritis:
The figure shows TNF stimulation causes Arid5a accumulation in the cytoplasm in a murine stromal fibroblast cell line untreated (left) and treated (right); Arid5a (red); RPL7A (green); nuclei (blue).
1Division of Rheumatology & Clinical Immunology, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
2BIOCEV, First Faculty of Medicine, Charles University, Vestec, Czech Republic.
3School of Medicine, Tsinghua Medicine, Tsinghua University, Beijing, China.