Genes influence when autism becomes visible, revealing early and late developmental patterns across childhood.



Off the coast of Antarctica, the sea ice retreated toward the southernmost continent and, like a bottle cap taken off a soda bottle, that reduced pressure slowed down a process of critical carbon dioxide capture, dramatically accelerating the warming of the planet.
But all that happened thousands of years ago, one of the death knells of the last ice age.
And yet, the sea ice of our own age is also retreating, so it’s critical that we understand these oceanic processes that have such a profound effect on the globe.
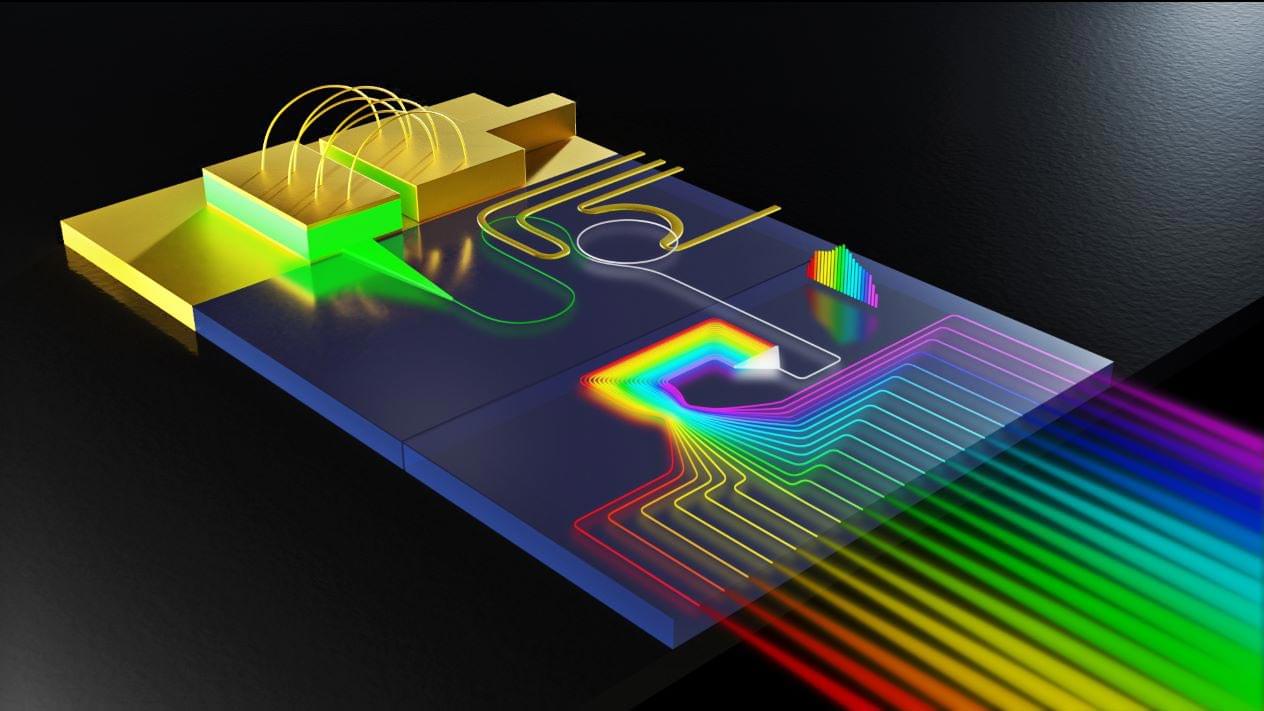
Defining the spatial organization of tissues and organs like the brain from large datasets is a major challenge. Here, authors introduce CellTransformer, an AI tool that defines spatial domains in the mouse brain based on spatial transcriptomics, a technology that measures which genes are active in different parts of tissue.
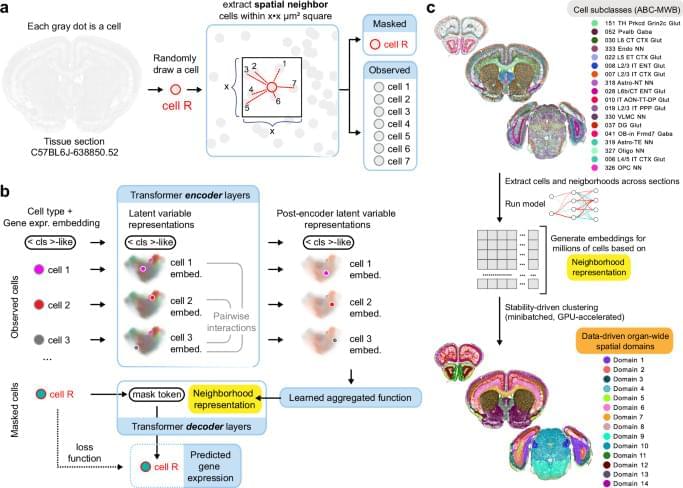
UCLA scientists, together with a team of international collaborators, have identified a promising new treatment strategy that can detect, kill and reprogram aggressive, treatment-resistant tumors like osteosarcomas and glioblastoma.
The findings, published in the journal Signal Transduction and Targeted Therapy, describe a novel approach that uses a specially engineered antibody, called DUNP19, to target a protein called LRRC15 that is found on the surface of certain aggressive cancer cells and the supportive stroma cells surrounding them. By pairing the antibody with radioactive particles, scientists can both visualize tumors for precise imaging and deliver targeted radiation therapy directly to cancerous tissue, while sparing healthy tissues.
When tested in mice, the LRRC15-targeted radionuclide therapy effectively slowed tumor growth, extended survival, and altered the tumor microenvironment to make it more receptive to an immune attack.
The suspected causes of Alzheimer’s disease are diverse, and its cures are, today, nonexistent.
What’s all but certain is that many who today have the mental chops to wade through a detailed article about the disorder’s drivers and demographics will nevertheless succumb to it someday.
With no cure available, despite numerous attempts to find one, researchers are looking down new roads for treatments. A recent discovery by Stanford Medicine neurologist Mike Greicius, MD, may help clear one of those roads for faster passage.

But the key is to see AI as fundamental, not a feature add. AI moves us from rule-based tasks to intelligent decision-making. When leaders rush to automate without analyzing and redesigning processes first, they implement smart tools on top of outdated workflows, expecting immediate gains.
I’ve seen it happen: When you apply AI to a flawed process, the consequences scale quickly. Instead, redesign workflows with AI at the core. Make sure the process makes sense before applying the technology.
Critically, when redesigning processes, understand that implementation is about leadership, not tools. IT teams often focus too much on tools and infrastructure without aligning automation efforts to clear business outcomes. Without engaging leadership and your workforce early and addressing cultural resistance, adoption stalls—even if the technology works perfectly.
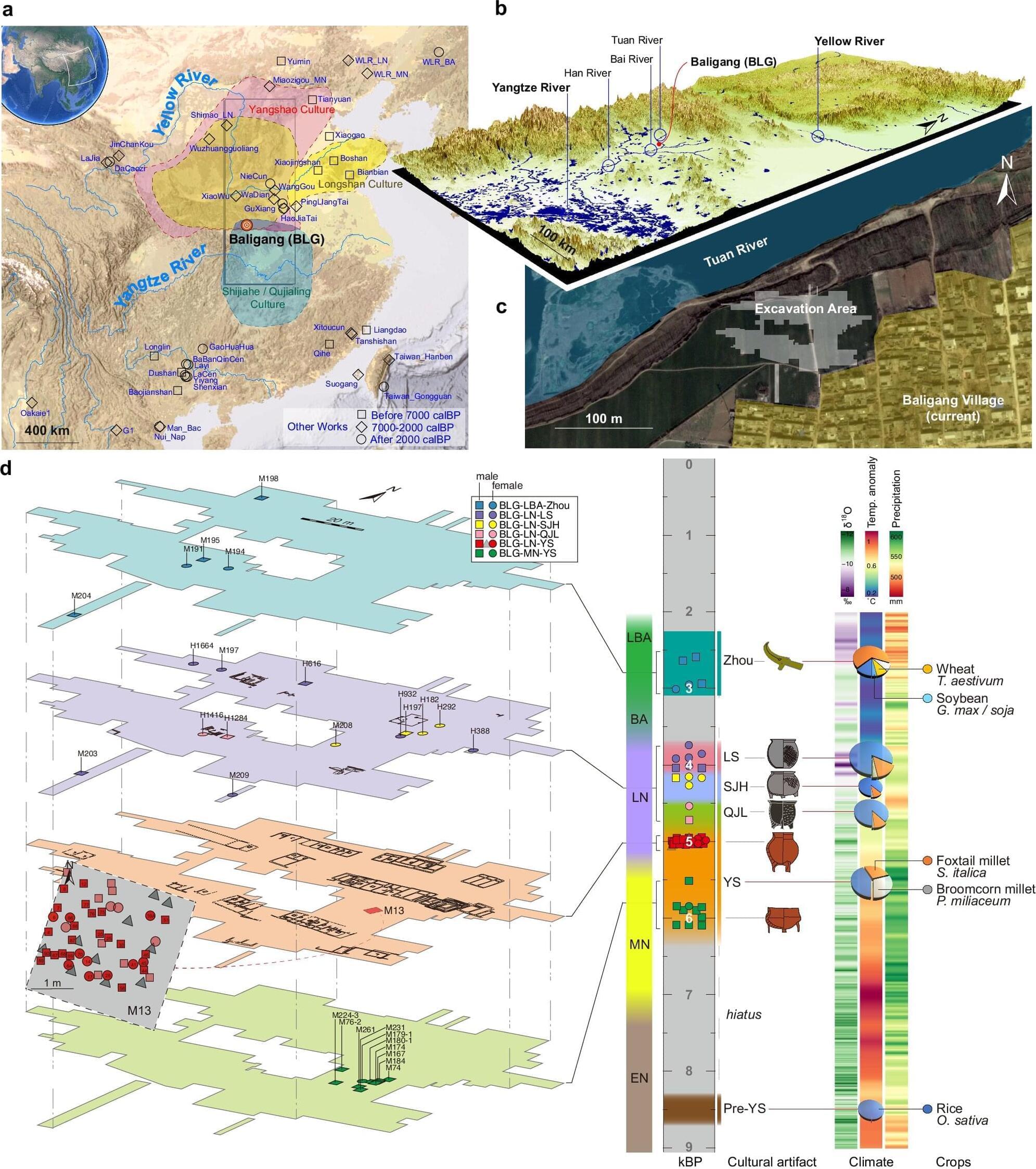
Scientists from Peking University have uncovered new genetic evidence that sheds light on how prehistoric people in China interacted, migrated, and built their communities. Led by Professors Huang Yanyi and Pang Yuhong from the Biomedical Pioneering Innovation Center (BIOPIC), the research reveals the first direct genetic proof of a patrilineal social system in Neolithic China.
The study, conducted in collaboration with Yunnan University and Minzu University of China, was published in Nature Communications on September 30, 2025.
The story of Chinese civilization begins along two great rivers: the Yellow River, known for its millet-farming cultures, and the Yangtze River, home to early rice agriculture. How people in these regions exchanged ideas, adapted to environmental shifts, and shaped early societies has long fascinated archaeologists and historians.
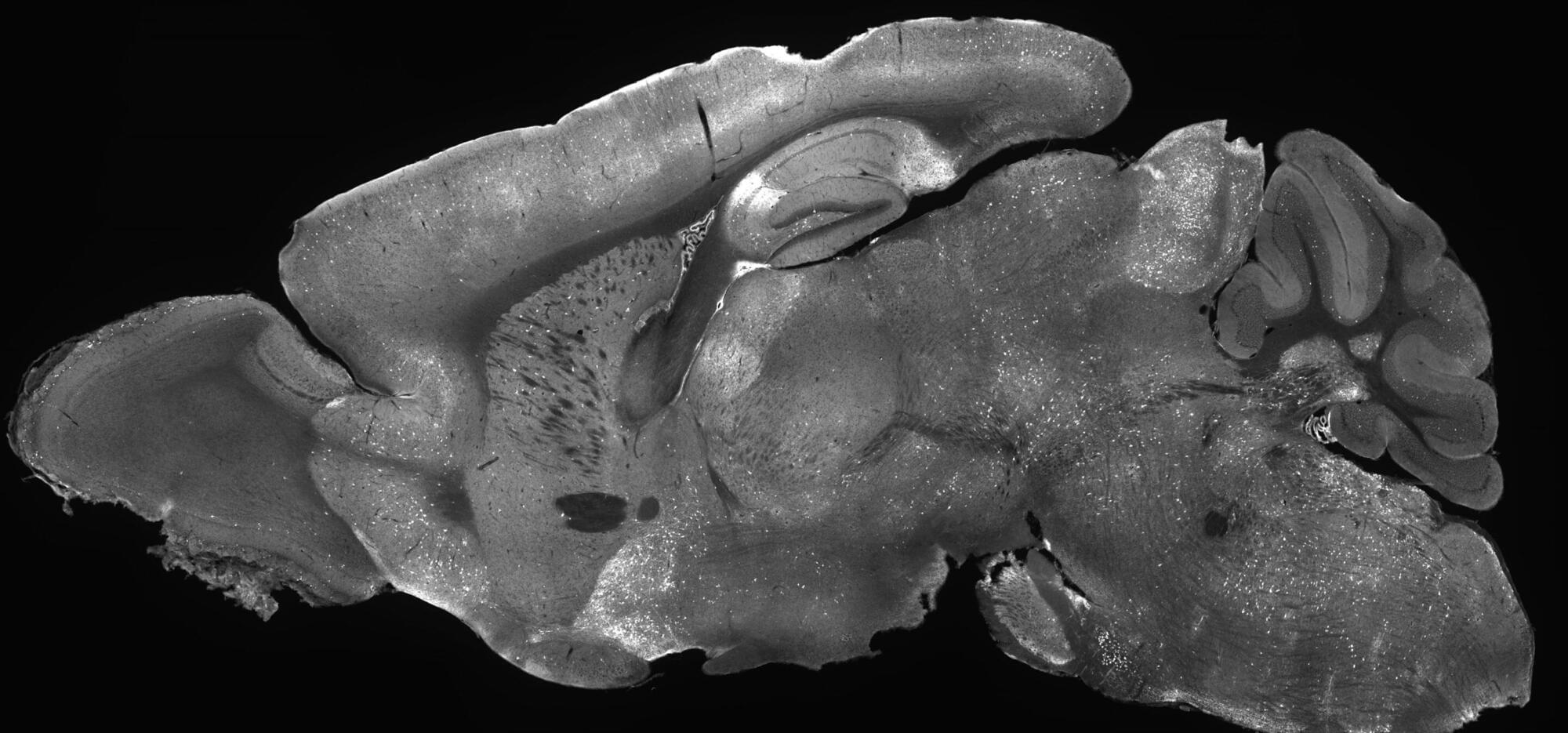
In an exciting scientific first, researchers at the Allen Institute successfully designed a new gene therapy that reversed symptoms related to SYNGAP1-related disorders (SRD) in mice. These are a class of brain disorders that can lead to severe and debilitating symptoms including intellectual disability, epilepsy, motor problems, and risk-taking behaviors in humans. In most cases, SRDs are caused when someone has only one working copy of the SYNGAP1 gene instead of the normal two.
The findings, recently published in the journal Molecular Therapy, represent the first successful gene supplementation therapy for SRDs in which an adeno associate virus (AAV) was used to deliver a working copy of the SYNGAP1 gene into brain cells. AAVs are non-replicating viruses that act like delivery trucks carrying therapeutic cargo, in this case the SYNGAP1 gene, into cells that need it.
“Gene supplementation is providing a functional new copy of a defective gene, a strategy that has great potential for correcting diseases where a gene is completely missing or where a single copy of a gene is lost,” said Boaz Levi, Ph.D., associate investigator at the Allen Institute and senior author of the study. “This provides a clear demonstration that SYNGAP1-related disorders can be treated with a neuron-specific gene supplementation strategy. It’s an important milestone for the field that provides hope for those who suffer from this class of severe neurological diseases.”
A study led by University of Massachusetts Amherst researchers demonstrates that their nanoparticle-based vaccine can effectively prevent melanoma, pancreatic and triple-negative breast cancer in mice. Not only did up to 88% of the vaccinated mice remain tumor-free (depending on the cancer), but the vaccine reduced—and in some cases completely prevented—the cancer’s spread.
The study is published in Cell Reports Medicine.
“By engineering these nanoparticles to activate the immune system via multi-pathway activation that combines with cancer-specific antigens, we can prevent tumor growth with remarkable survival rates,” says Prabhani Atukorale, assistant professor of biomedical engineering in the Riccio College of Engineering at UMass Amherst and corresponding author on the paper.