Futurism, transhumanism, bioethics, ethics, science, philosophy, artificial intelligence, personhood.



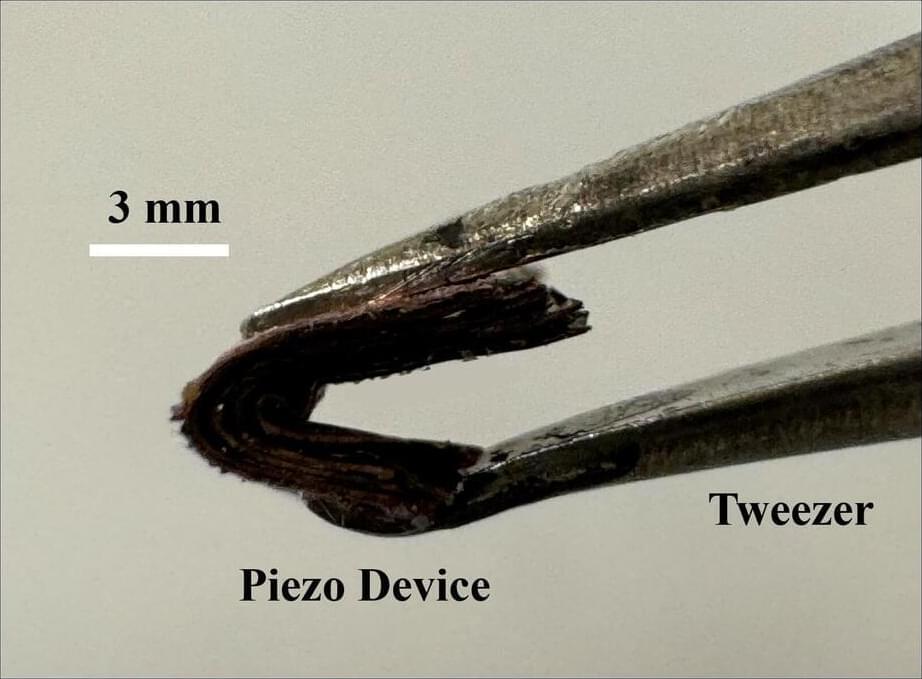
A new technology that can generate electricity from vibrations or even small body movements means you could charge your laptop by typing or power your smartphone’s battery on your morning run.
Researchers at the University of Waterloo have developed a tiny, wearable generator in response to the urgent need for sustainable, clean energy. It is also scalable for larger machines. Their paper, “Breaking Dielectric Dilemma: Polymer Functionalized Perovskite Piezocomposite with Large Current Density Output,” is published in the November edition of Nature Communications.
“This is a real game changer,” said Dr. Asif Khan, the project’s lead researcher and a postdoctoral fellow in the Department of Electrical and Computer Engineering at Waterloo. “We have made the first device of its kind that can power electronics at low cost and with unprecedented efficiency.”

And Patreon: https://www.patreon.com/nikitapetrov.
00:01:27 What is Russian cosmism?
00:12:51 The religious side of cosmism.
00:17:13 Cosmism as a response to the challenges of the 20th century.
00:25:20 Nature as a temporary enemy and eternal friend.
00:40:21 Pavel Florensky, the Russian da Vinci.
00:46:08 Plant life as a spiritual ideal.
00:51:17 The father of the Soviet space program, and his weird spirituality.
01:03:59 Cosmism and transhumanism: Compare and contrast.
01:09:01 Cosmism as a Russian propaganda project.
Watch this conversation on MeaningofLife.tv http://meaningoflife.tv/videos/36257
Nikita Petrov (MeaningofLife.tv) and George Young (University of New England, The Russian Cosmists)
Recorded on 09/06/2016.
And Patreon: https://www.patreon.com/nikitapetrov.
3:45 Does Russian thought have anything to contribute to the world?
15:30 Nikolay Fedorov, an eccentric thinker who influenced Tolstoy and Dostoyevsky.
24:17 Fedorov’s plan: Immortality for everyone.
31:48 ‘Kinship’ as a fundamental property of all matter.
45:13 A mass movement for the spiritual unity of humankind.
01:13:09 Vladimir Solovyov’s The Meaning of Love: Sexuality as a path to a Christian utopia.
01:36:27 Christ as the ultimate role model.
Watch this conversation on MeaningofLife.tv: http://meaningoflife.tv/videos/37671
Nikita Petrov (MeaningofLife.tv) and George Young (University of New England, The Russian Cosmists)
Recorded on December 7, 2016.

“Everyone thinks that [after] spinal injury, all you want to do is be able to walk again. But if you’re a tetraplegic or a quadriplegic, what matters most is working hands,” she said.
Reid received the device, called ARCex, as part of a 60-person clinical trial. She and the other participants completed two months of physical therapy, followed by two months of physical therapy combined with stimulation. The results, published today in Nature Medicine, show that the vast majority of participants benefited. By the end of the four-month trial, 72% experienced some improvement in both strength and function of their hands or arms when the stimulator was turned off. Ninety percent had improvement in at least one of those measures. And 87% reported an improvement in their quality of life.
This isn’t the first study to test whether noninvasive stimulation of the spine can help people who are paralyzed regain function in their upper body, but it’s important because a trial has never been done before in this number of rehabilitation centers or in this number of subjects, says Igor Lavrov, a neuroscientist at the Mayo Clinic in Minnesota, who was not involved in the study. He points out, however, that the therapy seems to work best in people who have some ability to move below the site of their injury.
Getting places in space quickly has been the goal of propulsion research for a long time.
Rockets, our most common means of doing so, are great for providing lots of force but extraordinarily inefficient. Other options like electric propulsion and solar sailing are efficient but offer measly amounts of force, albeit for a long time.
So scientists have long dreamed of a third method of propulsion – one that could provide enough force over a long enough time to power a crewed mission to another star in a single human lifetime. And that could theoretically happen using one of the rarest substances in the Universe – antimatter.


Researchers have doubled the number of known dark comets, identifying two distinct types: larger ones in the outer solar system and smaller ones in the inner solar system.
This discovery raises new questions about their origins and their role in delivering life-sustaining materials to Earth.
Dark Comet Discoveries