The jet is twice the length of our galaxy!




Leo P, a small galaxy and a distant neighbor of the Milky Way, is lighting the way for astronomers to better understand star formation and how a galaxy grows.
In a study published in the Astrophysical Journal, a team of researchers led by Kristen McQuinn, a scientist at the Space Telescope Science Institute and an associate professor in the Department of Physics and Astronomy at the Rutgers University-New Brunswick School of Arts and Sciences, has reported finding that Leo P “reignited,” reactivating during a significant period on the timeline of the universe, producing stars when many other small galaxies didn’t.
By studying galaxies early in their formation and in different environments, astronomers said they may gain a deeper understanding of the universe’s origins and the fundamental processes that shape it.

For decades there has been near constant progress in reducing the size, and increasing the performance, of the circuits that power computers and smartphones. But Moore’s Law is ending as physical limitations – such as the number of transistors that can fit on a chip and the heat that results from packing them ever more densely – are slowing the rate of performance increases. Computing capacity is gradually plateauing, even as artificial intelligence, machine learning and other data-intensive applications demand ever greater computational power.
Novel technologies are needed to address this challenge. A potential solution comes from photonics, which offers lower energy consumption and reduced latency than electronics.
One of the most promising approaches is in-memory computing, which requires the use of photonic memories. Passing light signals through these memories makes it possible to perform operations nearly instantaneously. But solutions proposed for creating such memories have faced challenges such as low switching speeds and limited programmability.

Xenon gas inhalation reduced neurodegeneration and boosted protection in preclinical models of Alzheimer’s disease. Most treatments being pursued today to protect against Alzheimer’s disease focus on amyloid plaques and tau tangles that accumulate in the brain, but new research from Mass General Brigham and Washington University School of Medicine in St. Louis points to a novel — and noble — approach: using Xenon gas. The study found that Xenon gas inhalation suppressed neuroinflammation, reduced brain atrophy, and increased protective neuronal states in mouse models of Alzheimer’s disease. Results are published in Science Translational Medicine, and a phase 1 clinical trial of the treatment in healthy volunteers will begin in early 2025.
“It is a very novel discovery showing that simply inhaling an inert gas can have such a profound neuroprotective effect,” said senior and co-corresponding author Oleg Butovsky, PhD, of the Ann Romney Center for Neurologic Diseases at Brigham and Women’s Hospital (BWH), a founding member of the Mass General Brigham healthcare system. “One of the main limitations in the field of Alzheimer’s disease research and treatment is that it is extremely difficult to design medications that can pass the blood-brain barrier — but Xenon gas does. We look forward to seeing this novel approach tested in humans.”
“It is exciting that in both animal models that model different aspects of Alzheimer’s disease, amyloid pathology in one model and tau pathology in another model, that Xenon had protective effects in both situations,” said senior and co-corresponding author David M. Holtzman, MD, from Washington University School of Medicine in St. Louis.
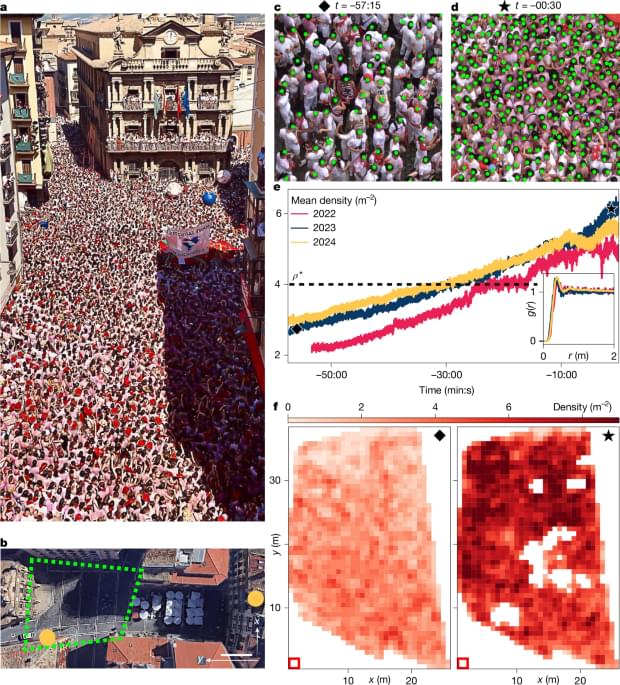
Dense crowds form some of the most dangerous environments in modern society. Dangers arise from uncontrolled collective motions, leading to compression against walls, suffocation and fatalities. Our current understanding of crowd dynamics primarily relies on heuristic collision models, which effectively capture the behaviour observed in small groups of people. However, the emergent dynamics of dense crowds, composed of thousands of individuals, remains a formidable many-body problem lacking quantitative experimental characterization and explanations rooted in first principles. Here we analyse the dynamics of thousands of densely packed individuals at the San Fermín festival (Spain) and infer a physical theory of dense crowds in confinement. Our measurements reveal that dense crowds can self-organize into macroscopic chiral oscillators, coordinating the orbital motion of hundreds of individuals without external guidance. Guided by these measurements and symmetry principles, we construct a mechanical model of dense-crowd motion. Our model demonstrates that emergent odd frictional forces drive a non-reciprocal phase transition7 towards collective chiral oscillations, capturing all our experimental observations. To test the robustness of our findings, we show that similar chiral dynamics emerged at the onset of the 2010 Love Parade disaster and propose a protocol that could help anticipate these previously unpredictable dynamics.
Humans are not yet done cooking. We’re continuing to evolve and adjust to the world around us, the records of our adaptations written in our bodies.
We know that there are some environments that can make us unwell. Mountain climbers often succumb to altitude sickness – the body’s reaction to a significant drop in atmospheric pressure, which means less oxygen is taken in with each breath.
And yet, in high altitudes on the Tibetan Plateau, where oxygen levels in the air people breathe are notably lower than lower altitudes, human communities thrive.
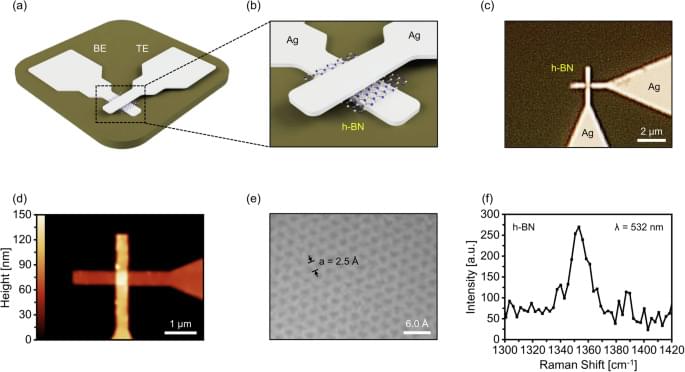
Yang, S.J., Jeon, YR., Kim, D. et al. npj 2D Mater Appl 9, 9 (2025). https://doi.org/10.1038/s41699-025-00533-9
UMD researchers have discovered key mechanisms in gene regulation that could improve the design of RNA-based medicines.
RNA-based medicines are among the most promising approaches to combating human disease, as evidenced by the recent successes of RNA
Ribonucleic acid (RNA) is a polymeric molecule similar to DNA that is essential in various biological roles in coding, decoding, regulation and expression of genes. Both are nucleic acids, but unlike DNA, RNA is single-stranded. An RNA strand has a backbone made of alternating sugar (ribose) and phosphate groups. Attached to each sugar is one of four bases—adenine (A), uracil (U), cytosine ©, or guanine (G). Different types of RNA exist in the cell: messenger RNA (mRNA), ribosomal RNA (rRNA), and transfer RNA (tRNA).