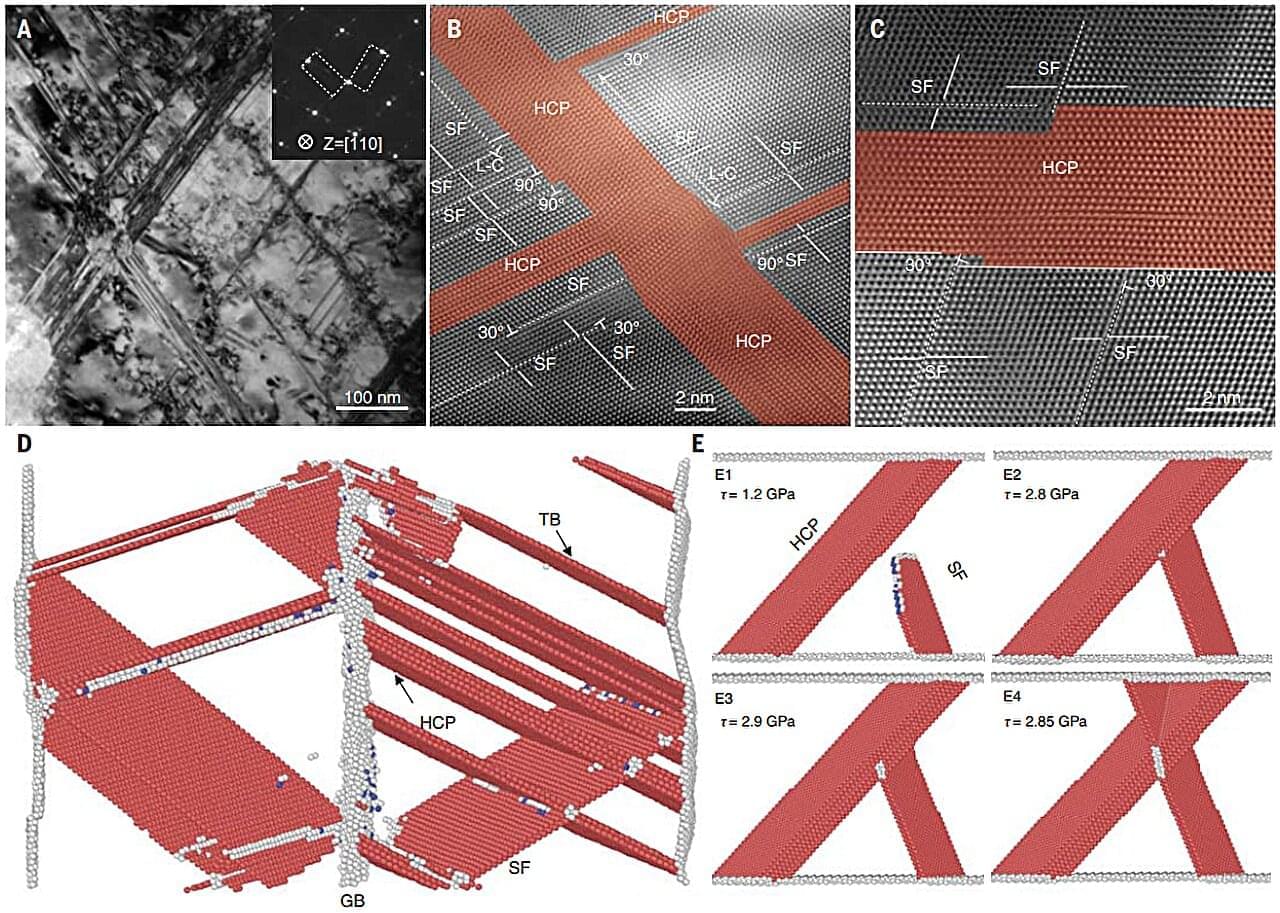
A combined team of metallurgists, materials scientists and engineers from the Chinese Academy of Sciences, Shandong University and the Georgia Institute of Technology has developed a way to make stainless steel more resistant to metal fatigue. In their study published in the journal Science, the group developed a new twisting technique that functions as an “anti-crash wall” in the steel, giving it much more strength and resistance to cyclic creep.
Metal can experience fatigue when bent many times, leading to breaking. When this occurs in critical applications, it can result in catastrophic accidents such as bridge failures. Because of that, scientists have for many years been working to reduce or prevent stress levels in metals. In this new effort, the researchers found a way to dramatically improve the strength of a type of stainless steel while also boosting its resistance to what is known as cycle creep, where fatigue occurs due to ratcheting, a form of repeated bending.
The new technique involved repeatedly twisting a sample of 304 austenitic stainless steel in a machine in certain ways. This led to spatially grading the cells that made up the metal, resulting in the build-up of what the team describes as a submicron-scale, three-dimensional, anti-crash wall. Under a microscope, the researchers found an ultra-fine, sub-10 nanometer, coherent lamellar structure that slowed dislocation by preventing stacking faults.