Model can complete tasks in under a second that take conventional methods hours


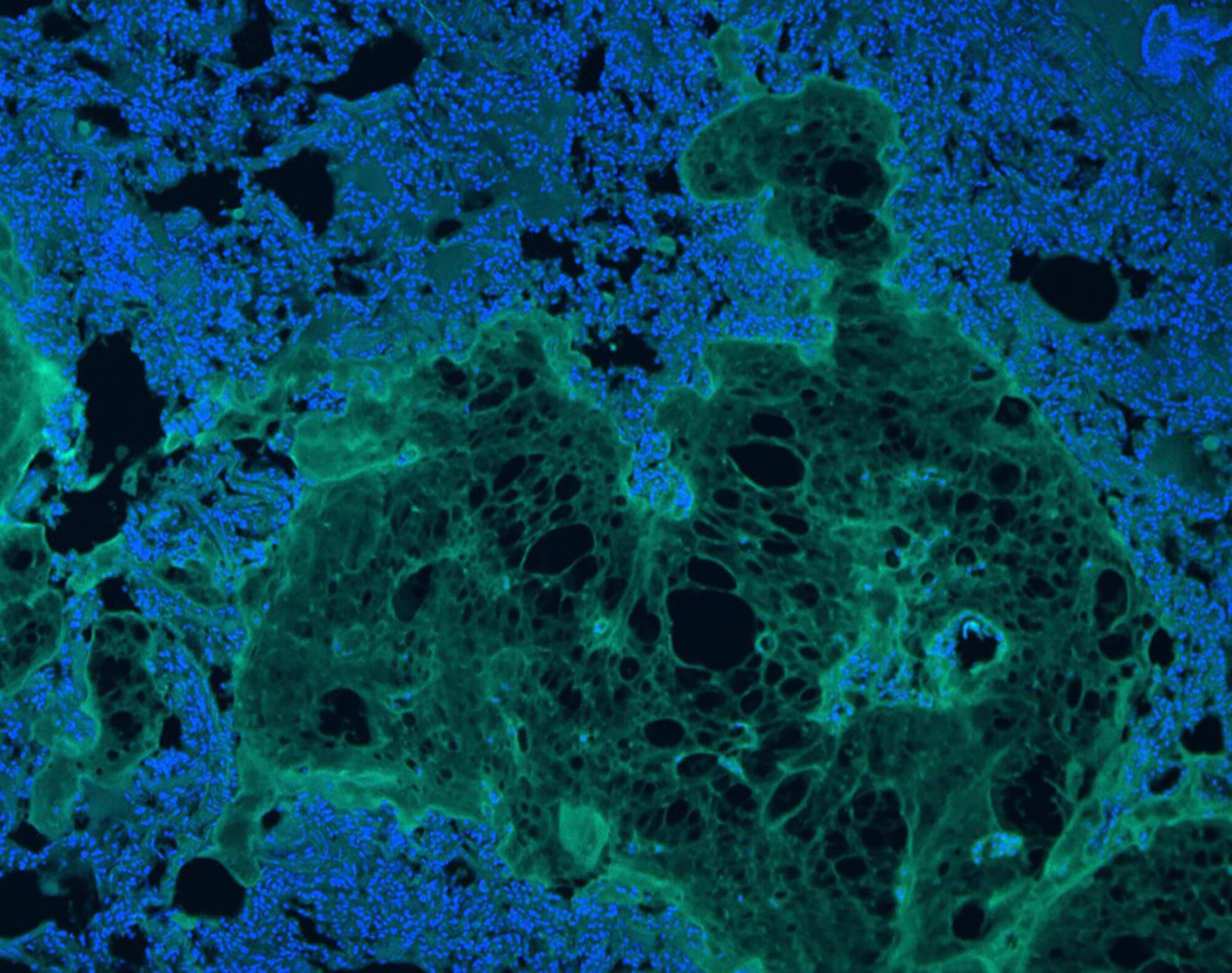
University of California, Los Angeles and University of California, San Diego researchers developed an injectable sealant for rapid hemostasis and tissue adhesion in soft, elastic organs.
Formulated with methacryloyl-modified human recombinant tropoelastin (MeTro) and Laponite silicate nanoplatelets (SNs), the engineered hydrogel demonstrated substantial improvements in tissue adhesion strength and hemostatic efficacy in preclinical models involving lung and arterial injuries.
Injuries to soft tissues such as lungs, heart, and blood vessels complicate surgical closure due to their constant motion and elasticity. Sutures, wires, and staples are mechanically fixed, risking blood loss when applied to tissues that expand and contract with each breath or heartbeat. Existing hemostatic agents, including fibrin-based sealants, aim to stem blood flow but may trigger intense coagulation responses in patients with clotting disorders.

Although glasses exhibit disordered atomic structures, X-ray and neutron scattering reveal a subtle periodicity. Researchers at the University of Tsukuba have demonstrated that this hidden periodicity—referred to as “invisible order”—plays a critical role in determining vibrational fluctuations in the terahertz (THz) frequency range, which significantly influence the physical properties of glass.
The research is published in the journal Scientific Reports.
At first glance, glass appears to be a random network of atoms. However, X-ray and neutron beam analysis reveals a faint but consistent periodic feature known as the first sharp diffraction peak (FSDP).
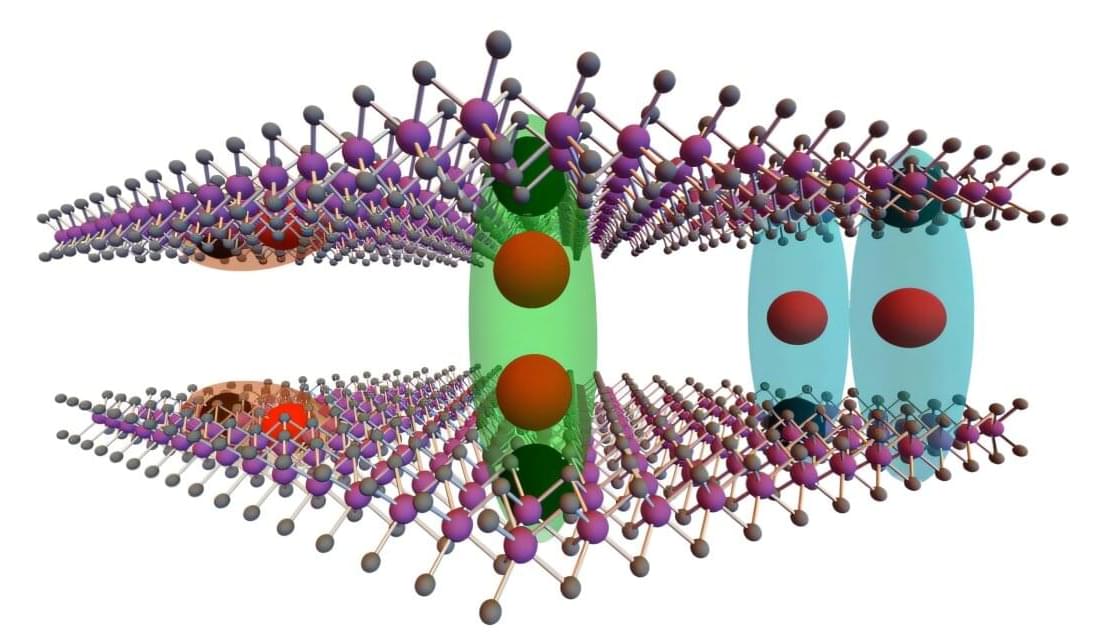
Two-dimensional (2D) materials have proved to be a promising platform for studying exotic quasiparticles, such as excitons. Excitons are bound states that emerge when an electron in a material absorbs energy and rises to a higher energy level, leaving a hole (i.e., the absence of an electron) at the site that it used to occupy.
Researchers at Heriot-Watt University and other institutes recently observed two distinct exciton states in bilayer molybdenum diselenide (MoSe₂) with a 2H-stacked configuration, which involves the alignment of two monolayers with a characteristic rotational symmetry. Their paper, published in Physical Review Letters, reports the observation of one of these states known as quadrupolar excitons in 2H-MoSe₂
“Our work was inspired by the ongoing effort to explore and control excitonic phenomena in atomically thin semiconductor materials, which are rich platforms for studying complex quantum states,” Mauro Brotons-Gisbert, senior author of the paper, told Phys.org. “In particular, bilayer transition metal dichalcogenides (TMDs) like MoSe₂ naturally host interlayer excitons with a dipolar character— bound states of electrons and holes residing in adjacent layers.”
Millions of years of evolution have enabled some marine animals to grow complex protective shells composed of multiple layers that work together to dissipate physical stress. In a new study, engineers have found a way to mimic the behavior of this type of layered material, such as seashell nacre, by programming individual layers of synthetic material to work collaboratively under stress. The new material design is poised to enhance energy-absorbing systems such as wearable bandages and car bumpers with multistage responses that adapt to collision severity.
Many past studies have focused on reverse engineering to replicate the behavior of natural materials like bone, feathers and wood to reproduce their nonlinear responses to mechanical stress. A new study, led by the University of Illinois Urbana-Champaign civil and environmental engineering professor Shelly Zhang and professor Ole Sigmund of the Technical University of Denmark, looked beyond reverse engineering to develop a framework for programmable multilayered materials capable of responding to local disturbances through microscale interconnections.
The study findings are published in the journal Science Advances.

Wearable technologies are revolutionizing health care, but design limitations in adhesive-based personal monitors have kept them from meeting their full potential.
A new University of Arizona study, published in Nature Communications, describes a longer-lasting, 3D-printed, adhesive-free wearable capable of providing a more comprehensive picture of a user’s physiological state.
The device, which measures water vapor and skin emissions of gases, continuously tracks and logs physiological data associated with dehydration, metabolic shifts and stress levels.
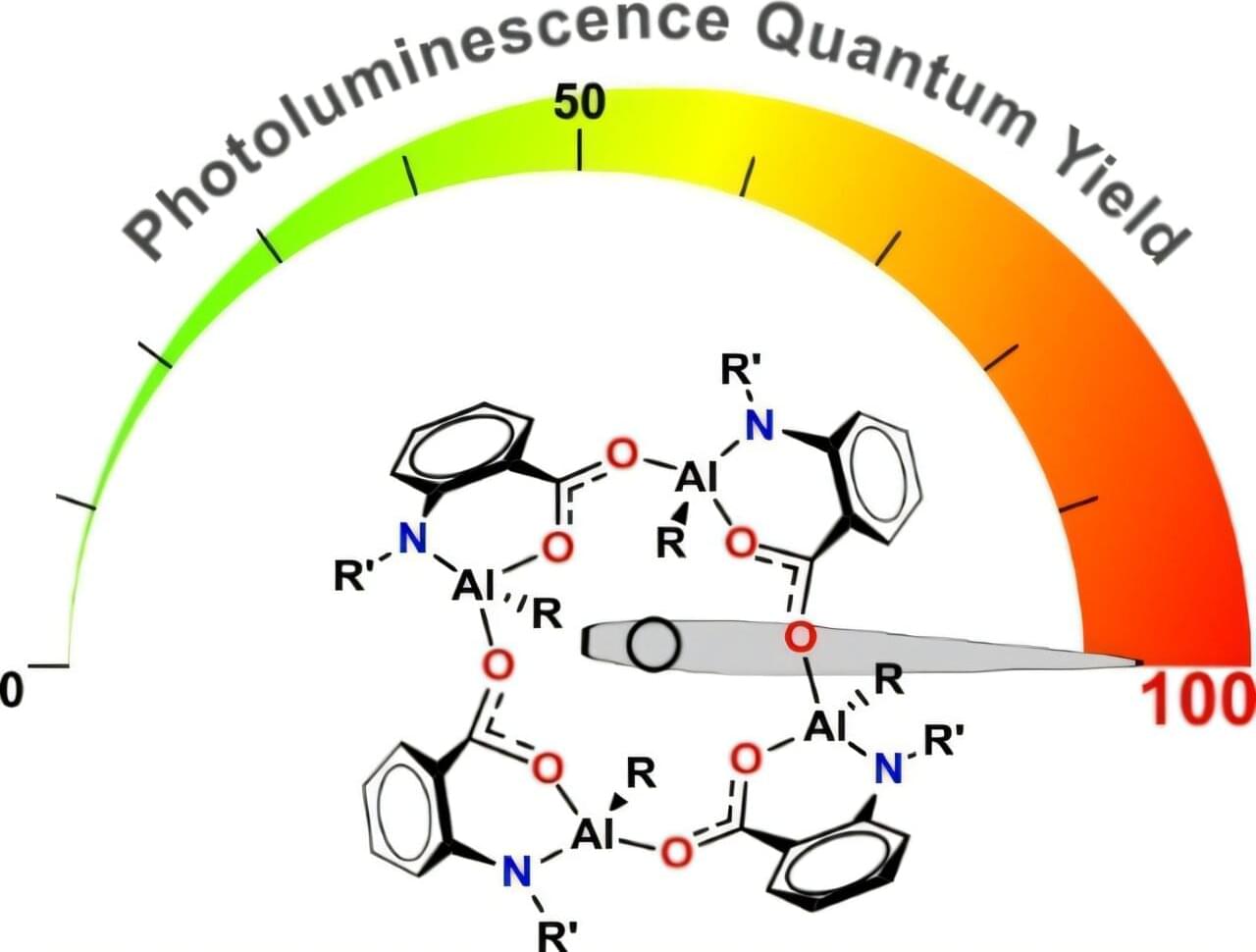
Artificial light, once a luxury, has become central to modern life, with its evolution spanning from fire to LEDs. Now, researchers have developed a new class of efficient light-emitting materials as promising candidates to be applied to lighten the darkness. They demonstrated easily accessible aluminum-based organometallic complexes that have the potential to be applied in optoelectronic devices.
The research team is from the Institute of Physical Chemistry, Polish Academy of Sciences in Warsaw and Warsaw University of Technology led by Prof. Janusz Lewiński in collaboration with Prof. Andrew E. H. Wheatley from Cambridge University. The paper is published in the journal Angewandte Chemie International Edition.
Growing demand for artificial light spurred the development of energy-efficient solutions like fluorescent lamps and, later, light-emitting diodes (LEDs). Once production costs dropped, LEDs became ubiquitous in homes and portable devices.