Simple and accompanied by definitions.


The behavior of these Technological Corporations are outside of our societal norms. This behavior lacks a level of enlightenment and freedom. Which as Transhumanists we should be against.
On May 2nd, the village idiots at Facebook made a decision to ban speech. Mainly, the decision was to ban the conservative voices of Alex Jones, Paul Joseph Watson, Milo Yiannopoulos, and Laura Loomer. This ban also impacted Louis Farrakhan, how he got lumped into this group is kind of funny, Facebook must have wanted to add a brown face to the white people who got banned. Thinking that this would be ok, because it is not just, white conservatives.
What I find is the biggest joke of this, is that the label that Facebook used was “Extremist” and “Dangerous” for these individuals and the groups that they represent. Which is not based on facts or proof, because I doubt if any of these individuals have a felony conviction.
The truth of the matter, this is a case of speech that should not be protected. Which is not Facebook’s job or any other groups job to control in the United States. People have a right to speak and have a right to their own points of view, without the fear of discrimination.


As he sits stroking his Rip Van Winkle-worthy beard, it’s easy to see how de Grey’s achieved this “kind of a spiritual leader-status,” as he calls it. He dives easily into intricate explanations of two research projects unfolding in the lab down the hall, eagerly describing how one studies mitochondrial mutations, which are thought to cause an increase in oxidative stress. The other looks at atherosclerosis, the narrowing and hardening of artery walls. If we understood more about this buildup, the logic goes, we could better clean it up before too much damage is done.
Though he attends lab meetings and oversees the SENS’s research, his primary task is convincing the general public that death is, in fact, bad and that we should be doing everything we can to stop it. This focus on messaging suits him just fine. “I’m not in this to do science for the sake of doing science,” he says. “I’m in it for the ultimate goal.” He does a “ridiculous” amount of media, he says, and gives around 50 talks a year, from Vietnam to the Czech Republic.
Back in April, at a San Francisco blockchain conference called Block 2 the Future, de Grey began his talk with a disclaimer: “I probably ought to start by emphasizing that I don’t know fuck-all about cryptocurrencies. I am really only here because I have apparently quite a significant fan base in this community, and I am delighted that I do.”

The farms take donated bodies and bury them or leave them on the surface to decompose. Researchers can also set up and study specific circumstances, for example by placing bodies in water or in a vehicle in the farm. The world’s first and most famous farm opened in 1981 in Knoxville, Tennessee; at least six more sites have opened in the United States. In recent years, researchers have set up body farms in Australia and the Netherlands, and Canada will open one this year.
Sites that allow the study of human remains have long existed in the United States and have started to appear recently in other countries.
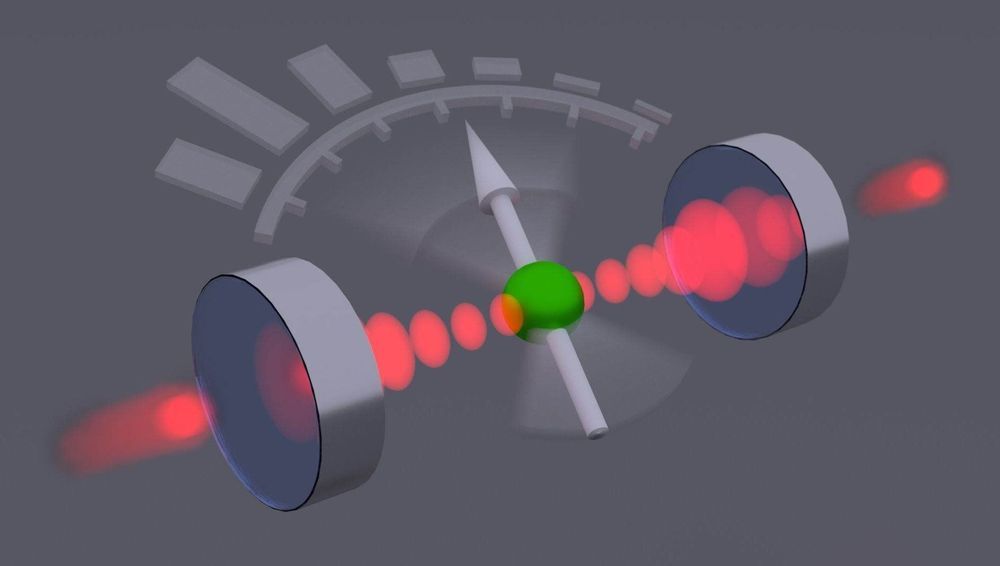
A photodetector converts light into an electrical signal, causing the light to be lost. Researchers led by Tracy Northup at the University of Innsbruck have now built a quantum sensor that can measure light particles non-destructively. It can be used to further investigate the quantum properties of light.
Physicist Tracy Northup is currently researching the development of quantum internet at the University of Innsbruck. The American citizen builds interfaces with which quantum information can be transferred from matter to light and vice versa. Over such interfaces, it is anticipated that quantum computers all over the world will be able to communicate with each other via fiber optic lines in the future. In their research, Northup and her team at the Department of Experimental Physics have now demonstrated a method with which visible light can be measured non-destructively. The development follows the work of Serge Haroche, who characterized the quantum properties of microwave fields with the help of neutral atoms in the 1990s and was awarded the Nobel Prize in Physics in 2012.
In work led by postdoc Moonjoo Lee and Ph.D. student Konstantin Friebe, the researchers place an ionized calcium atom between two hollow mirrors through which visible laser light is guided. “The ion has only a weak influence on the light,” explains Tracy Northup. “Quantum measurements of the ion allow us to make statistical predictions about the number of light particles in the chamber.” The physicists were supported in their interpretation of the measurement results by the research group led by Helmut Ritsch, a Innsbruck quantum optician from the Department of Theoretical Physics. “One can speak in this context of a quantum sensor for light particles”, sums up Northup, who has held an Ingeborg Hochmair professorship at the University of Innsbruck since 2017. One application of the new method would be to generate special tailored light fields by feeding the measurement results back into the system via a feedback loop, thus establishing the desired states.

Ambitious dreams have now become a reality as the Ocean Cleanup deploys its $20 million system designed to clean up the 1.8 trillion pieces of trash floating in the Great Pacific Garbage Patch. Check out another Forbes piece on how Ocean Cleanup aims to reuse and recycle the ocean plastic.


CHENNAI: As meteorologists observed a trough of low in the southern http://timesofindia.indiatimes.com/topic/Indian-Ocean”>Indian Ocean more than a week ago, five Indian satellites kept a constant eye on the system as it brewed into cyclone Fani.
As it developed into an “extremely severe cyclone”, the satellites launched by Isro sent data every 15 minutes to the ground station, helping track and forecast its movement and save hundreds of lives.
According to IMD, data from satellites Insat-3D, Insat-3DR, Scatsat-1, Oceansat-2 and Megha Tropiques was used to study the intensity, location and cloud cover around Fani. There was a cloud cover around the eye of the storm up to 1000km radius, though the rain clouds were only up to a radius of 100 to 200km. The rest were at a height of around 10,000feet.