Physicists from the University of Sussex have created what they called the tiniest microchips yet. The little microchips are made using graphene and other 2D materials and a form of “nano-origami.” The technique used in creating the tiny microchips marks the first time any researchers have been able to do this.
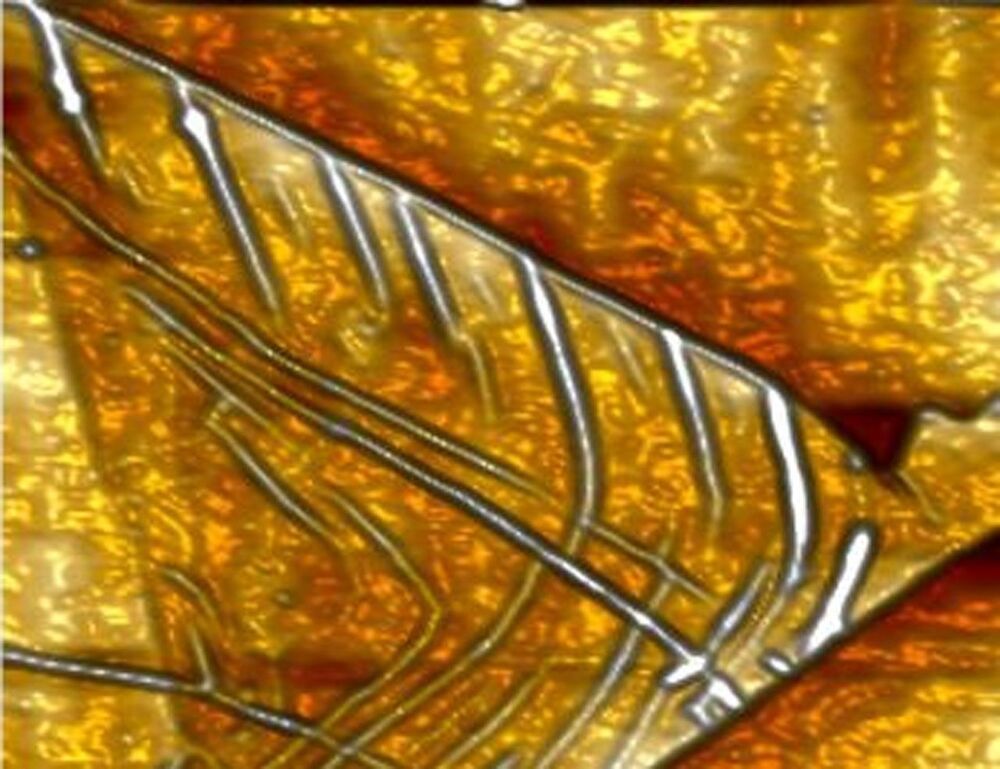
Researchers succeeded in making the tiny microchips by creating kinks in the structure of graphene to make the nanomaterial behave like a transistor. In their study, the team showed that when a graphene strip is crinkled in a specific way, it behaves like a microchip only about 100 times smaller than a conventional microchip. New construction methods are needed for microchips because traditional semiconducting technology is at the limit of what it can do.
The researchers believe that using the materials in their technique will make computer chips smaller and faster. The technology is dubbed “straintronics” and uses nanomaterials rather than electronics, allowing space for more chips inside a given device. The researchers believe everything we want to do with computers to speeding them up can be done by crinkling graphene.