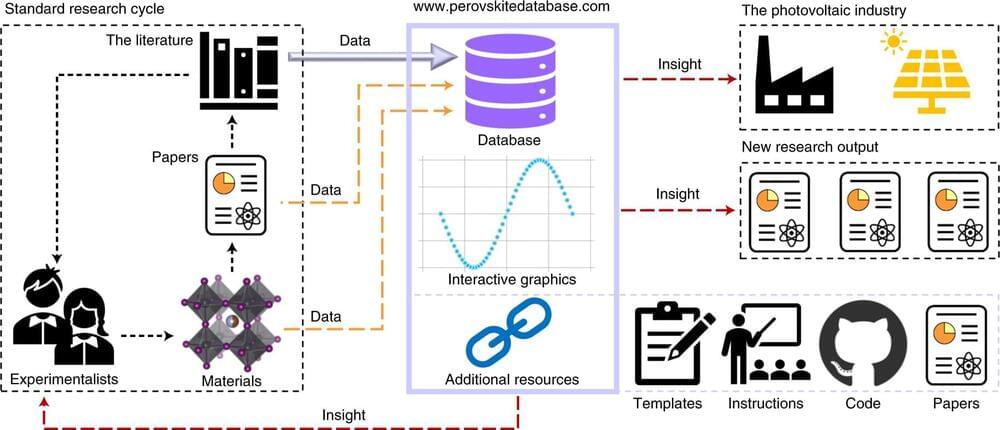
An international team of experts has collected data on metal halide perovskite solar cells from more than 15,000 publications and developed a database with visualization options and analysis tools. The database is open source and provides an overview of the rapidly growing knowledge as well as the open questions in this exciting class of materials. The study was initiated by HZB scientist Dr. Eva Unger and implemented and coordinated by her postdoc Jesper Jacobsson.
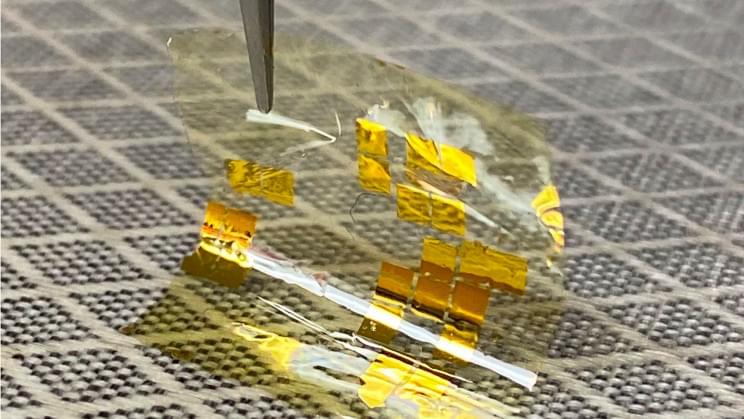
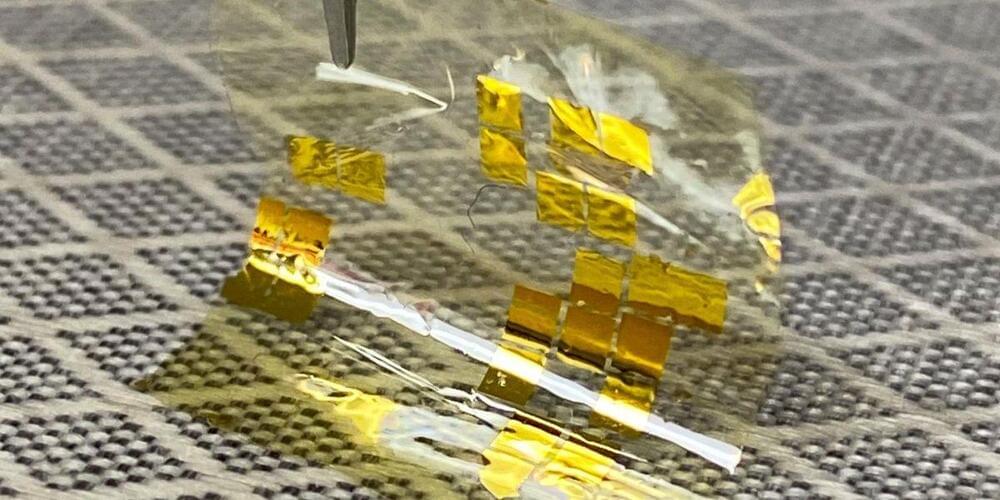
Halide perovskites have huge potential for solar cells and other optoelectronic applications. Solar cells based on metal-organic perovskites achieve efficiencies of more than 25 percent, they can be produced cheaply and with minimal energy consumption, but still require improvements in terms of stability and reliability. In recent years, research on this class of materials has boomed, producing a flood of results that is almost impossible to keep track of by traditional means. Under the keyword “perovskite solar,” more than 19,000 publications had already been entered in the Web of Science (spring 2021).
Now, 95 experts from more than 30 international research institutions have designed a database to systematically record findings on perovskite semiconductors. The data are prepared according to the FAIR principles, i.e. they are findable, accessible, interoperable and reusable. By reading the existing literature, the experts have collected more than 42,000 individual data sets, in which the data can be filtered and displayed according to various criteria such as material compositions or component type. Researchers from several teams at HZB were involved in this Herculean task.