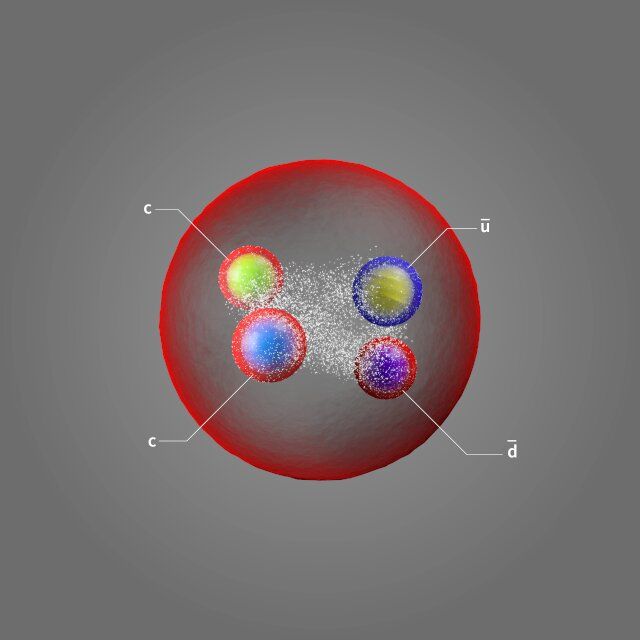
Quarks are the fundamental building blocks from which matter is constructed. They combine to form hadrons, namely baryons, such as the proton and the neutron, which consist of three quarks, and mesons, which are formed as quark-antiquark pairs. In recent years a number of so-called exotic hadrons—particles with four or five quarks, instead of the conventional two or three—have been found. Today’s discovery is of a particularly unique exotic hadron, an exotic exotic hadron if you like.
The new particle contains two charm quarks and an up and a down antiquark. Several tetraquarks have been discovered in recent years (including one with two charm quarks and two charm antiquarks), but this is the first one that contains two charm quarks, without charm antiquarks to balance them. Physicists call this “open charm” (in this case, “double open charm”). Particles containing a charm quark and a charm antiquark have “hidden charm”—the charm quantum number for the whole particle adds up to zero, just like a positive and a negative electrical charge would do. Here the charm quantum number adds up to two, so it has twice the charm!
The quark content of Tcc+, has other interesting features besides being open charm. It is the first particle to be found that belongs to a class of tetraquarks with two heavy quarks and two light antiquarks. Such particles decay by transforming into a pair of mesons, each formed by one of the heavy quarks and one of the light antiquarks. According to some theoretical predictions, the mass of tetraquarks of this type should be very close to the sum of masses of the two mesons. Such proximity in mass makes the decay “difficult,” resulting in a longer lifetime of the particle, and indeed Tcc+, is the longest-lived exotic hadron found to date.