Give science some understanding on Brilliant! First 200 to use our link https://brilliant.org/sabine will get 20% off the annual premium subscription. My favo…
Category: particle physics – Page 309

Polaritons open up a new lane on the semiconductor highway
On the highway of heat transfer, thermal energy is moved by way of quantum particles called phonons. But at the nanoscale of today’s most cutting-edge semiconductors, those phonons don’t remove enough heat. That’s why Purdue University researchers are focused on opening a new nanoscale lane on the heat transfer highway by using hybrid quasiparticles called “polaritons.”
Thomas Beechem loves heat transfer. He talks about it loud and proud, like a preacher at a big tent revival.
“We have several ways of describing energy,” said Beechem, associate professor of mechanical engineering. “When we talk about light, we describe it in terms of particles called ‘photons.’ Heat also carries energy in predictable ways, and we describe those waves of energy as ‘phonons.’ But sometimes, depending on the material, photons and phonons will come together and make something new called a ‘polariton.’ It carries energy in its own way, distinct from both photons or phonons.”

Time’ May Explain Why Gravity Won’t Play by Quantum Rules
A new theory suggests that the unification between quantum physics and general relativity has eluded scientists for 100 years because huge “fluctuations” in space and time mean that gravity won’t play by quantum rules.
Since the early 20th century, two revolutionary theories have defined our fundamental understanding of the physics that governs the universe. Quantum physics describes the physics of the small, at scales tinier than the atom, telling us how fundamental particles like electrons and photons interact and are governed. General relativity, on the other hand, describes the universe at tremendous scales, telling us how planets move around stars, how stars can die and collapse to birth black holes, and how galaxies cluster together to build the largest structures in the cosmos.
Quantum ‘magic’ could help explain the origin of spacetime
A quantum property dubbed “magic” could be the key to explaining how space and time emerged, a new mathematical analysis by three RIKEN physicists suggests. The research is published in the journal Physical Review D.
It’s hard to conceive of anything more basic than the fabric of spacetime that underpins the universe, but theoretical physicists have been questioning this assumption. “Physicists have long been fascinated about the possibility that space and time are not fundamental, but rather are derived from something deeper,” says Kanato Goto of the RIKEN Interdisciplinary Theoretical and Mathematical Sciences (iTHEMS).
This notion received a boost in the 1990s, when theoretical physicist Juan Maldacena related the gravitational theory that governs spacetime to a theory involving quantum particles. In particular, he imagined a hypothetical space—which can be pictured as being enclosed in something like an infinite soup can, or “bulk”—holding objects like black holes that are acted on by gravity. Maldacena also imagined particles moving on the surface of the can, controlled by quantum mechanics. He realized that mathematically a quantum theory used to describe the particles on the boundary is equivalent to a gravitational theory describing the black holes and spacetime inside the bulk.

Resolving the black hole ‘fuzzball or wormhole’ debate
Black holes really are giant fuzzballs, a new study says.
The study attempts to put to rest the debate over Stephen Hawking’s famous information paradox, the problem created by Hawking’s conclusion that any data that enters a black hole can never leave. This conclusion accorded with the laws of thermodynamics, but opposed the fundamental laws of quantum mechanics.
“What we found from string theory is that all the mass of a black hole is not getting sucked in to the center,” said Samir Mathur, lead author of the study and professor of physics at The Ohio State University. “The black hole tries to squeeze things to a point, but then the particles get stretched into these strings, and the strings start to stretch and expand and it becomes this fuzzball that expands to fill up the entirety of the black hole.”
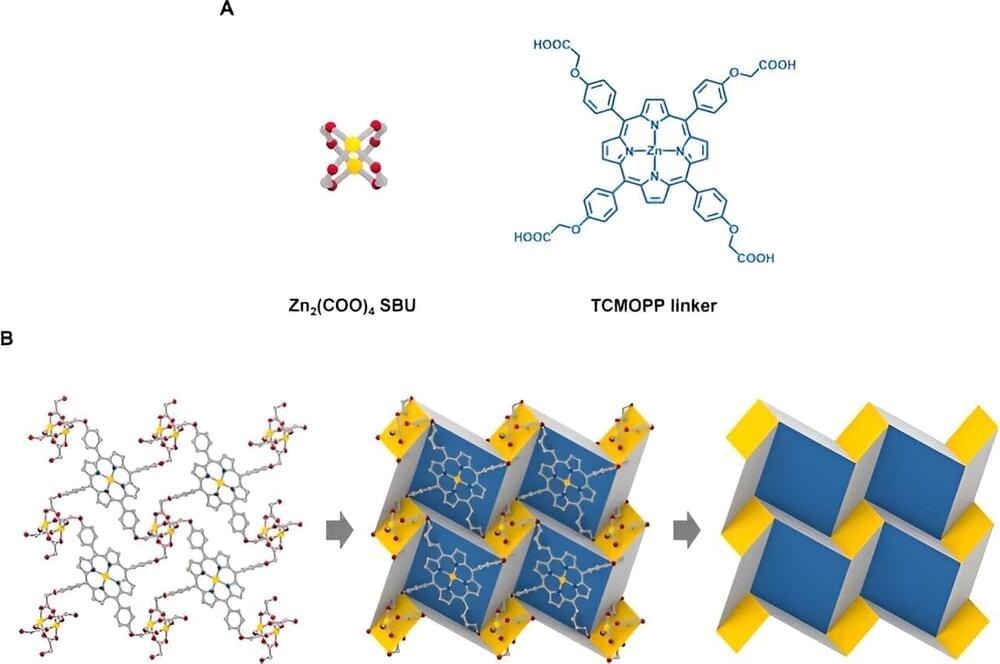
Metamaterials and origamic metal-organic frameworks
Origami is a paper folding process usually associated with child’s play mostly to form a paper-folded crane, yet it is, as of recently a fascinating research topic. Origami-inspired materials can achieve mechanical properties that are difficult to achieve in conventional materials, and materials scientists are still exploring such constructs based on origami tessellation at the molecular level.
In a new report now published in Nature Communications, Eunji Jin and a research team in chemistry and particle acceleration at the Ulsan National Institute of Science and Technology, Republic of Korea, described the development of a two-dimensional porphyrinic metal-organic framework, self-assembled from zinc nodes and porphyrin linkers based on origami tessellation.
The team combined theory and experimental outcomes to demonstrate origami mechanisms underlying the 2D porphyrinic metal-organic framework with the flexible linker as a pivoting point. The 2D tessellation hidden within the 2D metal-organic framework unveiled origami molecules at the molecular level.
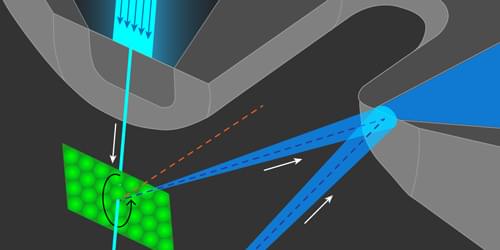
Atom Diffraction from a Microscopic Spot
Researchers have developed an atom-diffraction imaging method with micrometer spatial resolution, which may allow new applications in material characterization.
Microscopy with atoms offers new possibilities in the study of surfaces and two-dimensional (2D) materials [1]. Atom beams satisfy the most important requirements for microscopic probing: they can achieve high contrast and surface-specificity while doing little damage to the sample. A subtype of atomic microscopy—atomic-diffraction imaging—obtains measurements in reciprocal, or momentum, space, which is ideal for studying the surfaces of large and uniform crystalline samples. However, scientists developing this technique face challenges in achieving micrometer-scale spatial resolutions that would allow the study of polycrystalline materials, nonuniform 2D materials, and other surfaces without long-range order.

New dark matter theory explains two puzzles in astrophysics
Thought to make up 85% of matter in the universe, dark matter is nonluminous and its nature is not well understood. While normal matter absorbs, reflects, and emits light, dark matter cannot be seen directly, making it harder to detect. A theory called “self-interacting dark matter,” or SIDM, proposes that dark matter particles self-interact through a dark force, strongly colliding with one another close to the center of a galaxy.
In work published in The Astrophysical Journal Letters, a research team led by Hai-Bo Yu, a professor of physics and astronomy at the University of California, Riverside, reports that SIDM simultaneously can explain two astrophysics puzzles in opposite extremes.
“The first is a high-density dark matter halo in a massive elliptical galaxy,” Yu said. “The halo was detected through observations of strong gravitational lensing, and its density is so high that it is extremely unlikely in the prevailing cold dark matter theory. The second is that dark matter halos of ultra-diffuse galaxies have extremely low densities and they are difficult to explain by the cold dark matter theory.”